Current Research
For quicker navigation select one of the following
areas:
Heterogeneous
Catalysis
Research in our group is focused on gaining additional insight into zeolite catalytic processes in order to improve efficiency. We are particularly interested in demonstrating catalytic routes to new chemicals like pharmaceutical intermediates, specialty plastics and other consumer products like fragrances and artificial sweeteners. In order for zeolite catalysts to make an impact in these chemical markets the catalytic process must be very selective, i.e., high conversion to a single target molecule.
Green Chemistry describes the popular growing trend to reduce the use of
harmful solvents, eliminate the use of dangerous reagents and replace traditional
multi-step syntheses with cleaner more efficient reactions. This is
the major motivation for targeting the specialty chemical markets is to reduce
the use of dangerous chemicals like chlorofluorocarbons (CFCs) and deadly
reagents like HF or POCl2. For example, the pharmaceutical industry
is estimated to produce 75 mole equivalents of byproducts for every mole
of therapeutic compound. We hope to demonstrate zeolitic routes to
a few key intermediates with potential to improve the environmental impact
of the drug market.
Zeolite
Synthesis
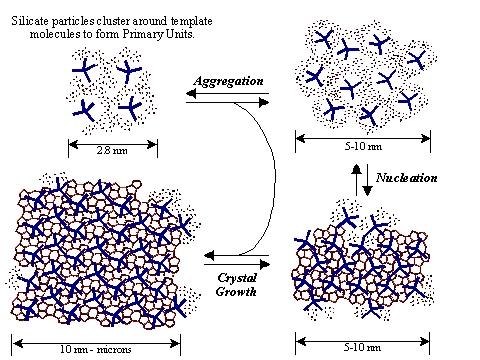
Clearly zeolite synthesis is not a mature field despite the variety of zeolite topologies and compositions. The primary structural feature of the materials that interests us is the low symmetry of the internal surface, e.g., undulating one-dimensional channels (IRF), intersecting channels with different diameters (CON). Such surfaces possess the unrealized potential to catalyze very selective, possibly asymmetric, reactions. The two examples cited were just recently discovered and their syntheses are far from optimized; the majority of the reported information is cited in patent literature. Synthetic goals in our research include the optimization of reported syntheses, variation of the composition of zeolites with known topologies and understanding the influence of synthetic conditions that favor the formation of these low-symmetry crystalline phases. Our zeolite synthesis investigations are vital and enable three key aspects of every research project in the group. First, we are able to investigate the newest, most promising classes of zeolite materials where there is no tenable source. Most of the synthetic zeolite phases are heavily protected industrial property. Second, the combination of high-resolution spectroscopy and catalytic investigations demands the highest level of sample purity, in reference to both crystallinity and composition. Even small amounts of paramagnetic metal impurities in the mineral sources used in the zeolite preparation will destroy local magnetic field homogeneity. Third, our expertise in materials synthesis enables us to design a material's composition in order to match the catalytic demands for a particular reaction study. For example, acid strength is highly dependent on both Si/M+ ratio and M+ distribution within the crystallite.
Solid-State
NMR Structures of adsorbates and reaction intermediates inside the porous cavities of the zeolite catalyst can be obtained using a number of high-resolution NMR techniques. Accurate distances can be obtained with state-of-the-art pulse sequences like REDOR, TRAPDOR, DRAWS and probe internuclear dipolar interactions. Information about the reactive, intermediate species provides details about the heterogeneous catalytic mechanism. Dynamic systems like chemical reactions are challenging systems to study, i.e., like hitting a moving target. NMR is uniquely capable of probing dynamics occurring from 10-6 to 1 s time-scale that covers many processes of interest. The NMR hardware in our lab is specialized to monitor chemical reactions under temperatures and pressures that simulate real reaction conditions. By monitoring the catalytic process in real-time we observe the sequence of events that occur as adsorbates are transformed to important products. 
|