|
|
|
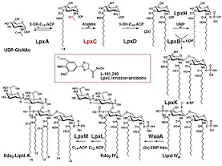
The cell wall
of gram-negative bacteria is surrounded by an outer membrane that is
comprised of charged lipopolysaccharide (LPS) molecules. (See Figure)
The highly charged outer membrane serves to prevent entry of
hydrophobic molecules into the bacteria. Lipid A is the hydrophobic anchor of LPS and is
essential for bacterial survival.
As a consequence, the enzymes in the Lipid A biosynthetic pathway are
obvious targets for the development of antimicrobial agents. The enzyme LpxC catalyzes the
deacetylation of UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc, the committed step
in the biosynthesis of Lipid A.
Inhibitors of the bacterial enzyme LpxC have been demonstrated to
have antimicrobial activity, validating LpxC as a drug target. The resistance to available
antibiotics creates a need for new antibiotics with activity against novel
targets. The development of
highly potent and specific inhibitors of LpxC will require a full
understanding of the enzyme's catalytic mechanism. LpxC has been demonstrated to be a
zinc-dependent deacetylase.
The focus of our research in this area is on further elucidating the
catalytic mechanism of this bacterial enzyme using classical biochemical as
well as novel chemical biology methods.
|
|
Bacterial Biosynthesis of
the Cell wall component Kdo2-Lipid A
|

|
Raetz, C.R.H., and Whitfield, C. Lipopolysaccharides endotoxins. Annu. Rev. Biochem. (2002) 71:
p. 635-700
|

|
Two zinc bound active site
of LpxC
|

|