Chem 125 - Experiment II
Solution Color
Experiment II - Solutions & Dilutions
Goals of Experiment II
Questions you should learn from this lesson and know before going into lab
Questions you should learn in lab
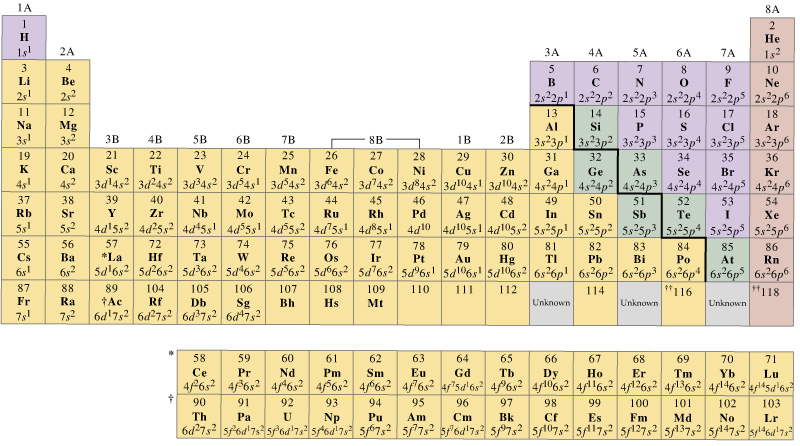
You should also have a general understanding of the periodic table.

When looking at the relationship of color of a solution, and the elements themselves there are certain characteristics in the periodic table that should be known. Charge, electron configuration, ionic radius, all of these are characteristics of the cation in a particular solution that may have an impact on color.
Think you know the periodic table? Test yourself to find out!!

Need some additional help with understanding the periodic table? Try these great links!
Webelements
PTable
Experiment Goal
Preparing a solution of known concentration
Preparing a solution is something that is very critical to many applications in science, medicine, cooking, engine matainence, and other fields. Particularly in chemistry, solutions are made using the concentration concept of molarity. You will go through the different concepts related making a solution, and go through a step by step use of calculating molarity.
Terms you will need to know for the experiment
Atomic Weight
Molecular Weight
Mole
Molarity
Volume
Concentration
Concepts you will learn
What is a mole?
What is molarity?
Why are volume and concentration important to making a solution?
Skills you will learn
How to determine molecular weight
How to weigh material
How to calculate molarity
How to work with volumetric flasks
Preparing a solution of known concentration
What is a mole?
The first thing you will need to understand when making a solution is the concept of a mole. A mole is a number 6.02 x 1023 to be exact. All chemistry calculations are calculated in moles. The concept of a mole is just like the concept of a dozen. There are 12 objects in a dozen, just like there are 6.02 x 1023objects in a mole. When working with different elements, they all have different atomic weights.
The atomic weight is how many grams of that element will make up one mole (or 6.02 x 1023 atoms) When this is applied to a ionic or molecular compound, the molecular or formula weight of the compound is determined by combining the atomic weight of all the atoms in the compound. The atomic weights for each atom can be found on any periodic table.
The first thing you should ALWAYS do is convert grams to moles. If you are given something in grams, then determine the formula weight and find out how many moles it is. All chemistry calculations are done with moles because they are numbers not weights. When baking, you do not weigh out 173 grams of egg, you would use 1 egg
Do you really know what a mole is? Test yourself below!

Preparing a solution of known concentration
Volume & Concentration
When you're making a solution of a given compound, you need to know what concentration you want, and what volume you want. These are important because they relate to how much of your compound you need.
You will also need to find the molecular weight of the compound like you did on the first page.
The volume of your container is important, because if there is a larger volume, you need more material.
Test yourself!
Show/hide comprehension question...
The next important step after you select a volume is the concentration
The concentration is the amount of something in a given container.
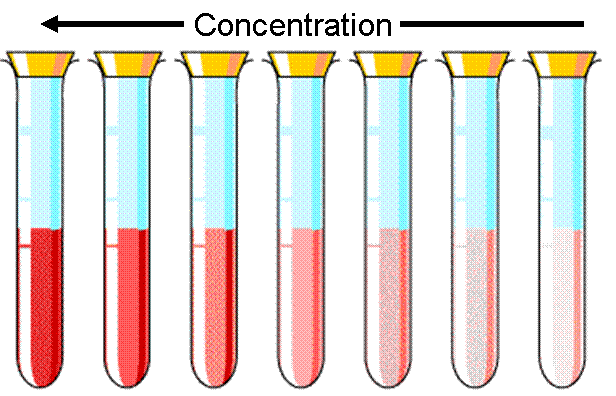
The higher the concentration, the more of the sample there is.
Moving from right to left in this picture the concentration is increasing, and you can see the solutions getting darker.
Show/hide comprehension question...
An example of concentration is stated below
There are 36 eggs in 3 cartons, so the concentration is 12eggs/carton
The graph below shows how the concentration changes as you remove eggs from the cartons. There are 3 cartons of 12 eggs to start with. 36eggs/3 cartons. The concentration is 12eggs/carton. As you remove eggs from the cartons, the concentration goes down. There are still 3 cartons, but now less eggs.

Preparing a solution of known concentration
Molarity
Molarity is a measurement of concentration.
Specifically for molarity, it is the number of moles in a given volume
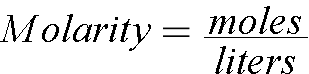
There are 6 moles of NaCl in 3 liters of water, so the Molarity (Concentration) is 2 moles / liter. The molarity of the soluion is 2.0
The main equation for calculating molarity is that molarity = the number of moles in one liter of solution
The video below shows exactly how to setup and use the molarity equation to determine the number of moles needed to make 100mL of a 0.1M solution
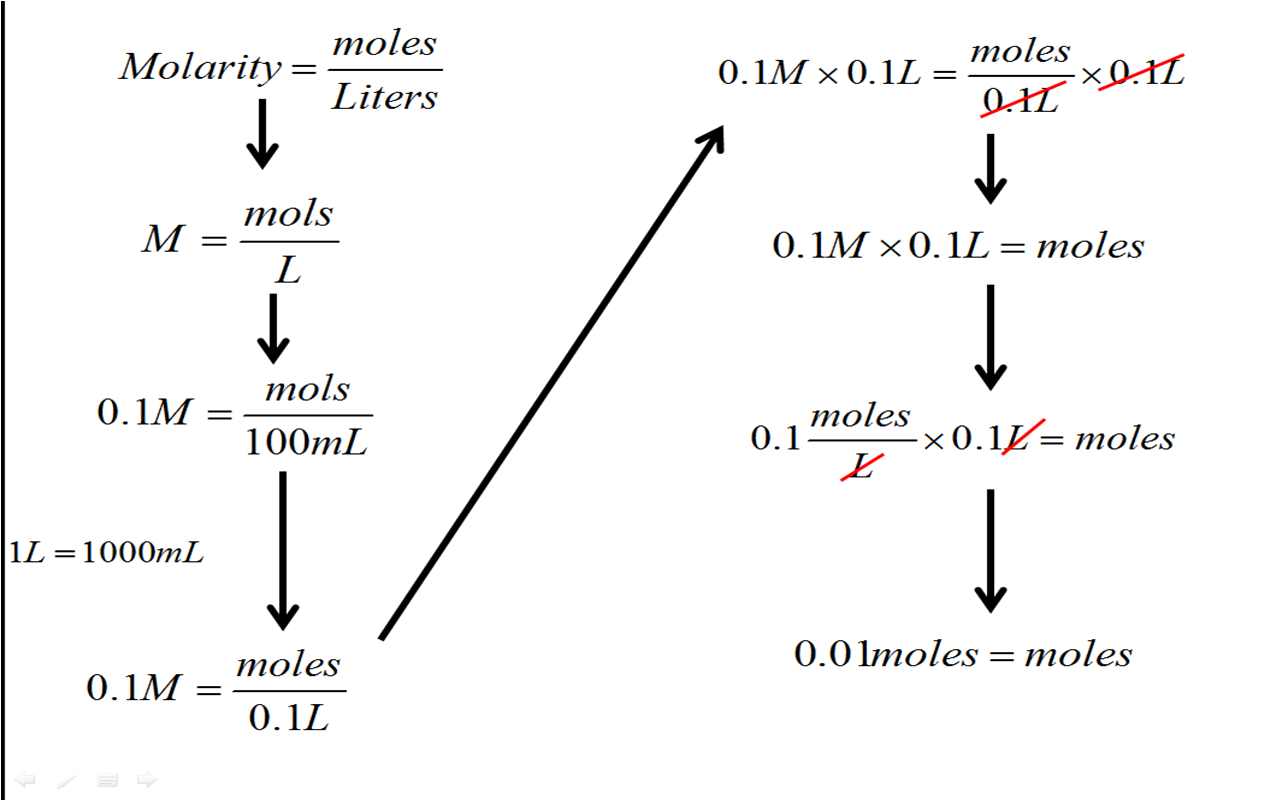
Now use the equation in the video to solve these problems. You may need to determine the molecular weight of compounds as well, so have your periodic table ready!

Still wanting some extra practice on calculating molarities, and volumes and moles? Visit the link below for a bottomless molarity worksheet!
Bottomless Worksheet of Molarity!
Preparing a solution of known concentration
Laboratory Details
Now that you're able to calculate molarity and know volumes and concentrations, let's take a look at what you will do in lab.
1). Preform calculations to determine how many grams of your assigned samples you need to weigh out to pepare 100mL of a 0.1M solution
See the previous page for help!!
2). Actually weigh out the amount of material you need for each solution. The video below will show you how to accurately weigh out solid chemicals.
Show/hide comprehension question...
Show/hide comprehension question...
3.) Next you will dissolve your solid chemical in the appropriate solvent.
Check To Make Sure You Use The Appropriate Solvent!!
Using the video below, try and answer the following questions.

4). You have now made a solution!!
Experiment Goal
Preforming a Solution Dilution
Preforming a dilution is a skill that is needed in just about every area where solutions are involved. In the medical field, concentrations of medicine are very important, but are generally kept as a stock solution at a higher concentration and would therefore need to be diluted prior to giving it to the patient. In certain engines, solutions of oil and gasoline are used and concentrations are critical to engine preformance so diluting from a higher concentration to a lower concentration is used quite a bit. All fields of chemistry and biology use solutions and their dilutions in the lab and in the field, so knowing how to prefom a dilution is a very important skill.
Terms you will need to know for the experiment
Dilution
Concentration
Molarity
Volume
Concepts you will learn
What is a dilution factor?
How do you work with ratios?
Skills you will learn
How to dilute samples
How to use a buret
Preforming a Solution Dilution
Solution Dilution & the Math Behind it
Now that you've prepared your solutions, you next will need to be able to dilute them and make solutions of a lower concentration.
Dilution measurments use the equation:
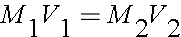
Where M1 is the molarity of the first solution and M2 is the molarity of the second, and V1 and V2 are the volumes.
This is actually a condensed equation of two molarity equations. Let's walk through how it comes to this.
You want to make 50mL of a 0.03M solution. You have a 0.1M solution.
We can start with the Molarity Equation
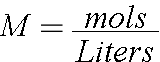
Start with what you want. You want 50mL of a 0.03M solution. You have a volume and a concentration so you can find how many moles of your compound will be in there. Try doing this!
Scroll here to check your answer!
Now that you know the number of moles you need, now you need to find a volume of your current solution, that has that many moles in it. Your current solution is 0.1M So how many mL would you need, to have that many moles?
Scoll here to check your answer!
Now that you found how many mL of your stock solution you need, how many mL of water will you need to add?
Scroll here to check your answer!
That was the long way of doing this problem. You can also use the simplified equation of
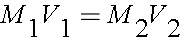
You want 50mL of a 0.03M solution. You have a volume and a concentration for one of the solutions, so that will go on one side
M1 = 0.03M
V1 = 50mL
You also have the concentration of the other solution. You have a stock solution of 0.1M
M2 = 0.1M
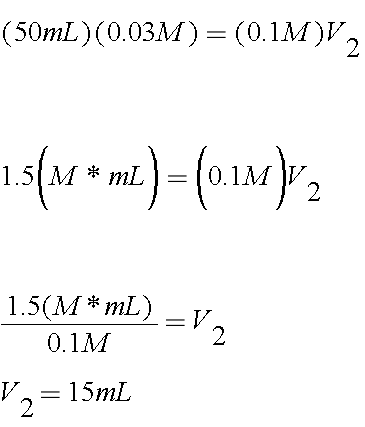
Now that you've worked through a problem, try some additional practice problems!

Preforming a Solution Dilution
Laboratory Details
When you're actually in the lab, then you can do these calculations yourselves on real samples. Many times though, you can decide for yourself what the volumes you need are. 1mL, 10mL, 20mL, 100mL Just remember, you have to be able to measure the volumes needed accurately. To do that, you will use your burets.
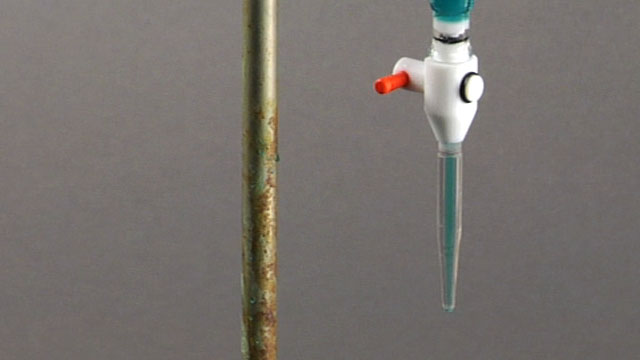
This is what a CLOSED buret looks like. When filling the buret, make sure that it is closed so that what you pour in, doesn't pour right out!
When you are making your dilutions, you will need two (2) burets, one for you initial stock solution, and one for your solvent (whatever you used to make your stock solution, e.g. water, acid, ammonia)
The video below shows exactly what you will do in lab.
Now try to answer these questions about the video. You may need to watch it again.

You've completed the tutorial on preparing a solution
& performing a dilution
Prior to the lab, you will also need to complete your prelab report, which will test your understanding of the following concepts
Determining molecular weight
Determining molarity
Calculating concentrations based on dilution factors
Hopfully when going into the lab, you are able to know the following skills
How to use a balance and weigh out a solid sample
How to calculate Molarity
How to use a volumetric flask
How to use a buret
If there are questions please attend the GSI office hours or contact Prof. Kerner. There is also a second part to this Experiment 2 online tutorial dealing with Beer's Law, absorbance, spectrophotometry, calibration curves, and determination of an unknown concentration.
Good Luck in Lab!