
Chem 125 - Experiment II
Solution Color
Experiment II - Solution Color, Absorbance, and Beer's Law
Goals of Experiment II
Questions you should learn from this lesson and know before going into lab
Questions you should learn in lab
Experiment Goal
Obtaining an Absorbance Spectrum
In obtaining an absorbance spectrum, you are getting a graph representation of how light is interacting with a solution, and how that relates to the solution color. This interaction is very important in scientific and medical fields and that color can give a lot of information. The color of a solution can give information on concentration of chemicals, how much acid is present, if a reaction has happened, or even if something has gone bad or not. In the following pages, you will learn how obtain an absorbance spectrum for each of the samples you prepared, and use that spectrum to relate to why the solution is the color that it is.
Terms you will need to know for the experiment
Light
Color
Wavelength
Absorbance
Transmittance
Concepts you will learn
How does light interact with a solution?
What types of light will a solution absorb or transmit?
How are absorbance and transmittance related?
How do absorbance and transmittance relate to the color of the solution?
Skills you will learn
How to use a spectrophotometer
How to obtain an absorbance spectrum
How to use that absorbance spectrum to relate to solution color
Obtaining an Absorbance Spectrum
Solution Color
Watch the video below which shows how to prepare a solution.
The solution made was Cu(NO3)2 where the cation was Cu2+. You will notice that the solid was blue, and the solution it made in water, was a blue color. Many times when you look at data in the CRC Handbook, it gives you the color of the SOLID form of the compound.
Match the following solid colors with the solution colors you would expect.
Show/hide comprehension question...
Did you match the right colors?
There are many things that can change a soluiton color. Many of them are based on the placement within the periodic table, and how it is set up.
Another aspect that can effect the color of a solution is the charge of the cation. Take a look at the video below.
Notice that the Vanadium solutions are all different colors, but they are all the same cation. The difference is that the cation has a DIFFERENT charge, which changes the electron configuration, and how light interacts with the solutions. This will be very important for future experiments involving redox reactions, as you are changing the charge of the cations. More on that later!
When you're in lab, look at the solutions that you make. Some questions you can ask yourself as you're making them are:
Obtaining an Absorbance Spectrum
Light & Solutions
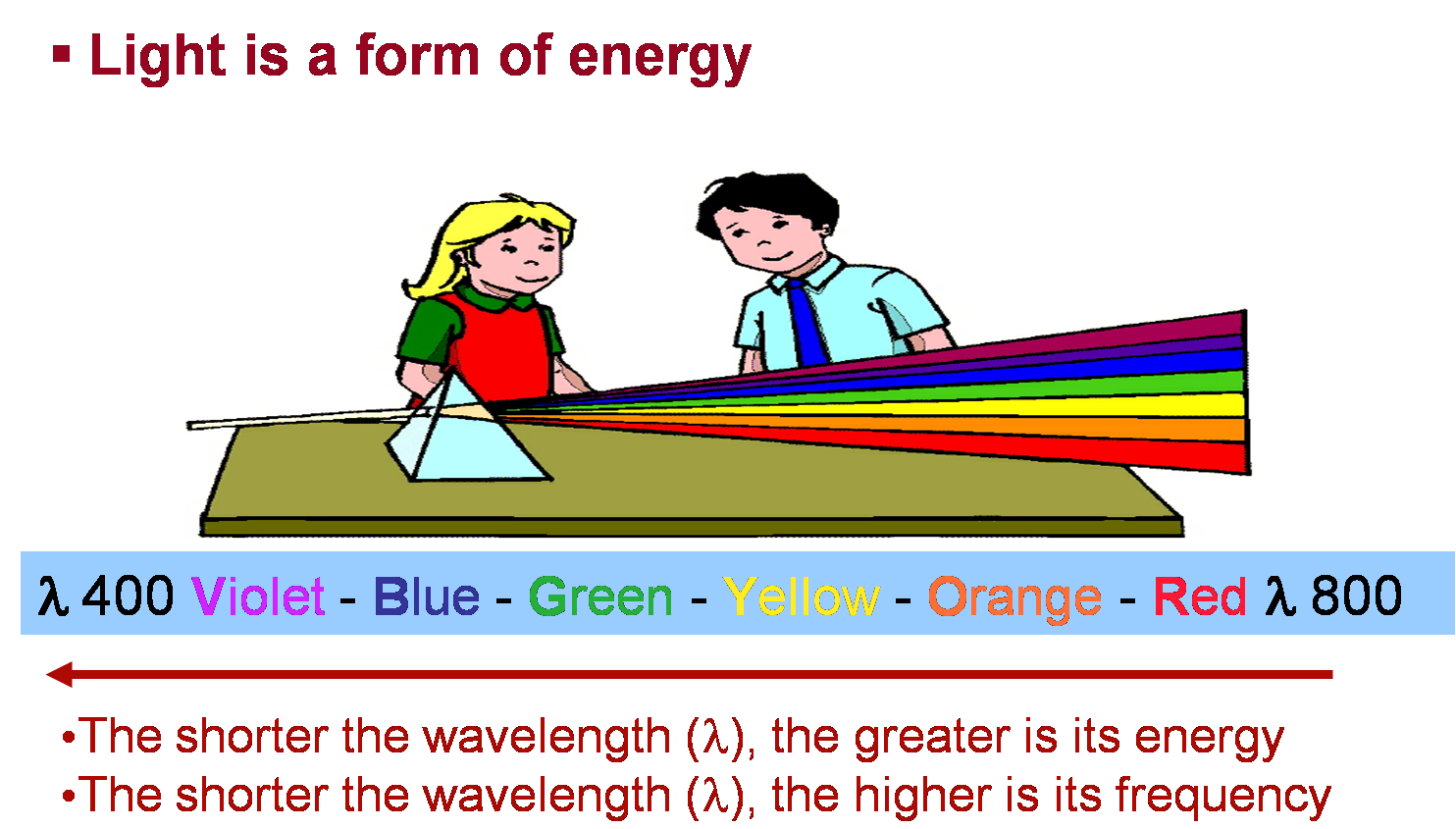
Most of the light that all of you see, like the light from the sun, is white light. But did you know that the light coming from the sun is made up of many different colors?
It's time you are introduced to Mr Roy G. Biv, the keeper of the colors!!

White light is primarily made up of these seven colors, but the color is different at each wavelength.
You will see the individual colors of each of these wavelengths.
Bright White Light!
When you look at a solution, there is light from all around interacting with it. This is how you are able to see the soluton color.
Much of the time, you are seeing how things interact with white light. This light is the light that comes from the sun, and many light bulbs.
It is comprised of all the colors of the rainbow (ROY G. BIV)!!! But what does that have to do with solution color? The color of a solution depends on how the light (of many colors) interacts with the solution, and is a combination of colors from that rainbow!
Below shows how white light would interact with a sample that you have.
1). All the colors of light in white light, hit the solution.
2). Each wavelength of light interacts with the molecules in the solution.
3). Some colors of light are blocked by the molecules and Absorbed, while others pass through and are Transmitted
A questions to consider:
What do the transmitted colors represent?
If you have a sample of a blue solution, what color light would be the most transmitted?
Obtaining an Absorbance Spectrum
The Absorbance Spectrum
You've looked at what light does all at once, but for many things, including this experiment, the light is broken down into wavelengths. Each wavelength has its own color. When you see how each wavelength of light interacts with the solution on its own, you can generate an absorbance spectrum (graph) for your solution.
Each wavelength can absorb differently, below is an example of how that happens.
1). The different colors of light hit the solution one at a time.
2). Each color of light interacts with the molecules and some part of the light is Absorbed, while the rest is Transmitted.
3). The amount of light that is transmitted is different for each wavelength.
4). The amount of light that is Transmitted passes through the solution to the detector.
5). The machine then can convert the amount of light that is Transmitted into the amount of light that was blocked or Absorbed by the solution.
As the light interacts with the sample, you can ask yourself.
Once the light interacts with your solution, you can graph it, much like this spectrum of Chlorophyll A
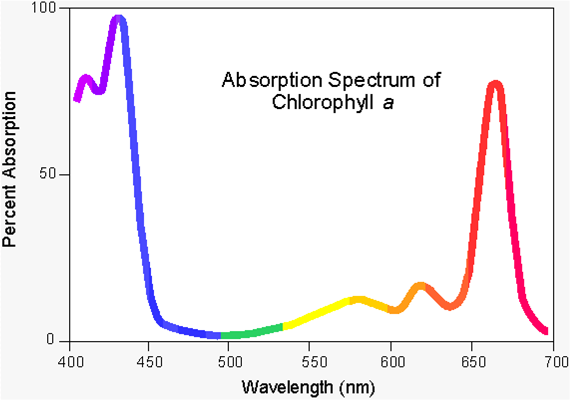
Try this Activity below with an absorbance spectrum

A question to think about:
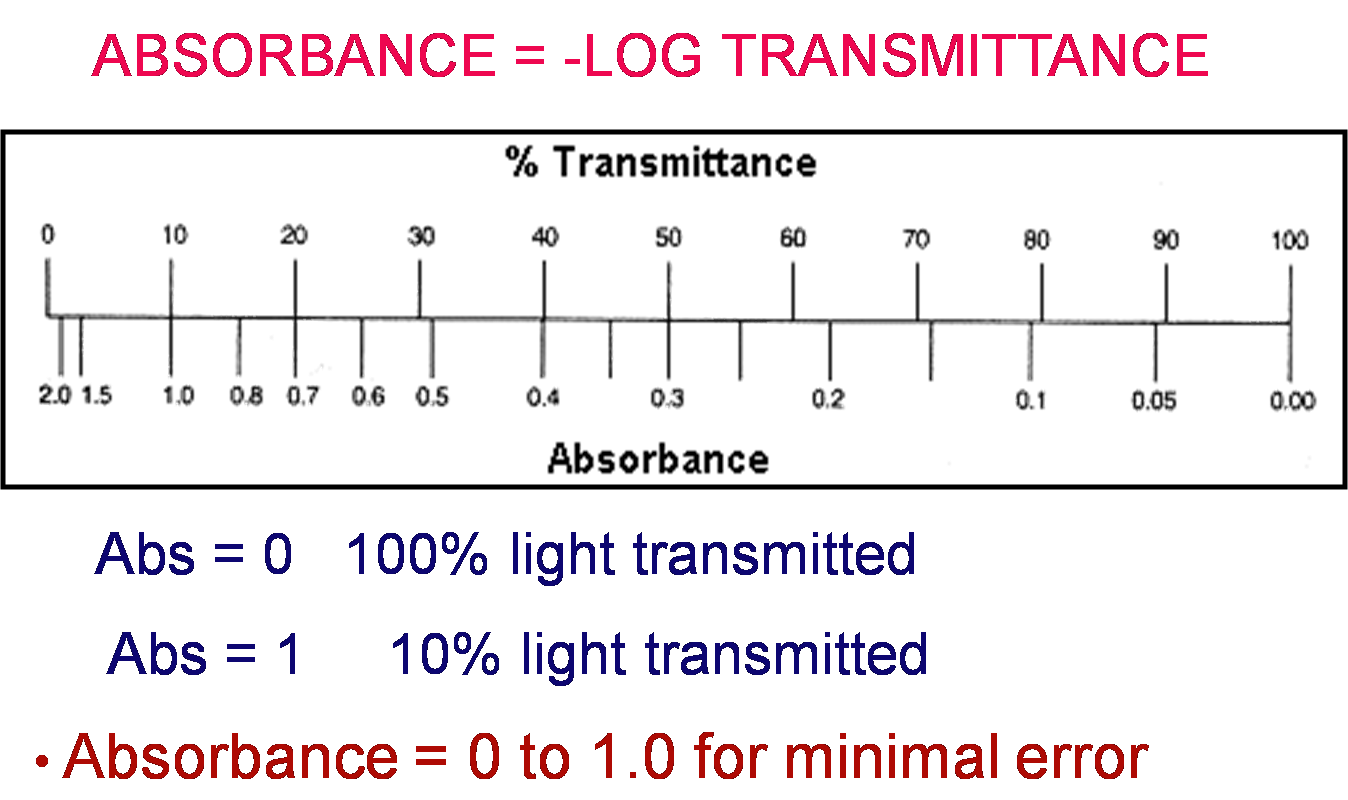
Absorbance vs. Transmittance
Absorbance and Transmittance are related. As one goes up, the other goes down
Absorbance is the amout of light that is blocked by sample molecules
Transmittance is the amout of light that passes through the solution.
But HOW are they related?
Obtaining an Absorbance Spectrum
Laboratory Details
You will take each of the solutions you already made, and create an absorbance spectrum for each one. To do this you will use the spectrophotometer to select a wavelength of light and measure and record the absorbance of the sample at that wavelength.
Use the labeled picture below to guide youself through the steps for taking an absorbance measurement.

1). Tune the spectrophotometer to the desired wavelength
2). Load a cuvette with the solvent used in your sample
What did you use to make your solution? Water? Acid? Base? This is your solvent, and this is what you will use to tare the spectrophotometer.
Make sure the clear sides of the cuvett are in the direction of the light.
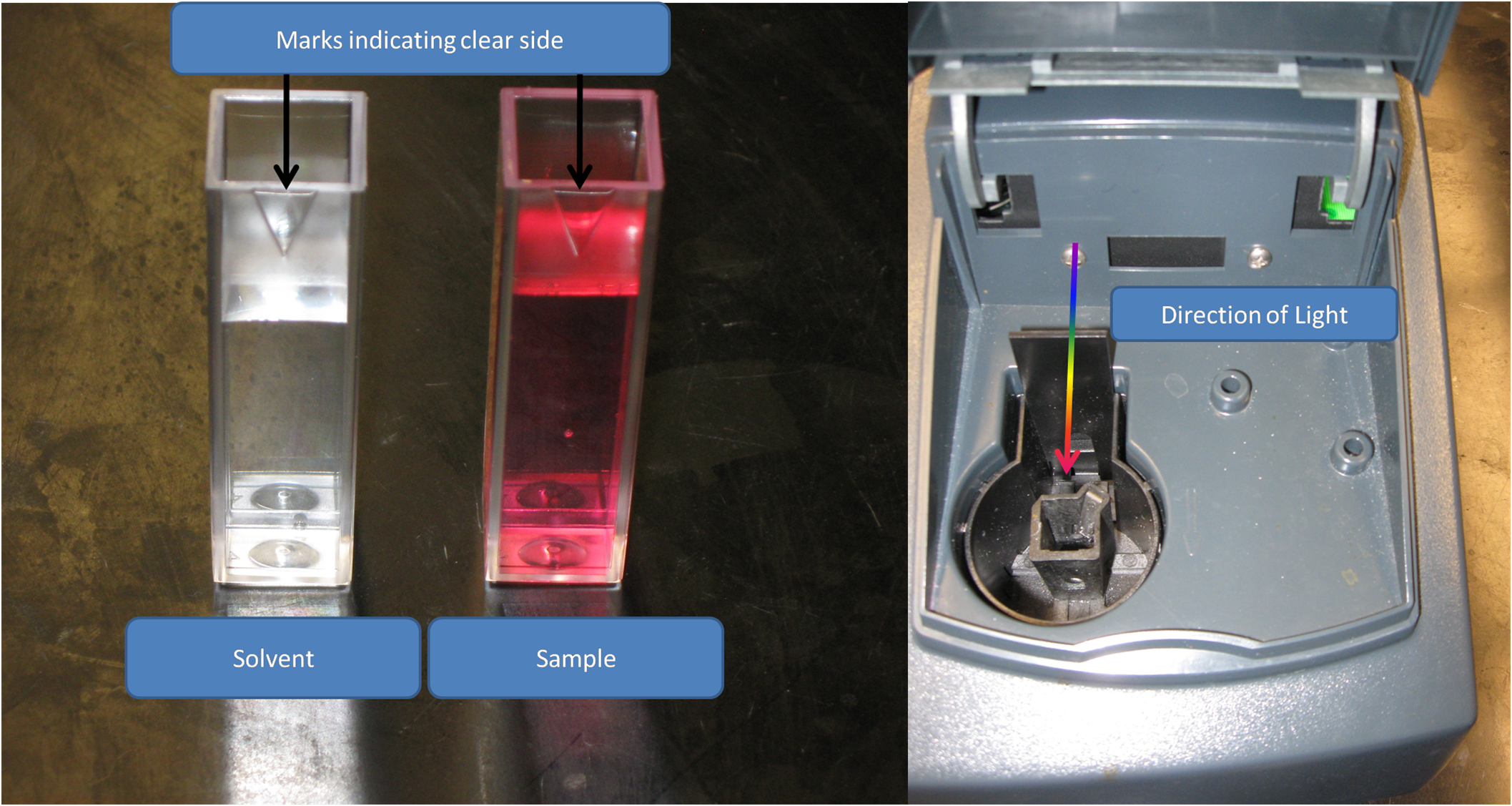
3). Place the cuvette in the spectrophotometer, and close it. Then tare the spectrophotometer.
This is just like taring a balance. You are telling the spectrophotometer that whatever light it is measuring, should be equal to zero absorbance.
4). Once the spectrophotometer is tared, then remove the solvent cuvette, and load a cuvette with your sample. Record the absorbance value that it gives you.
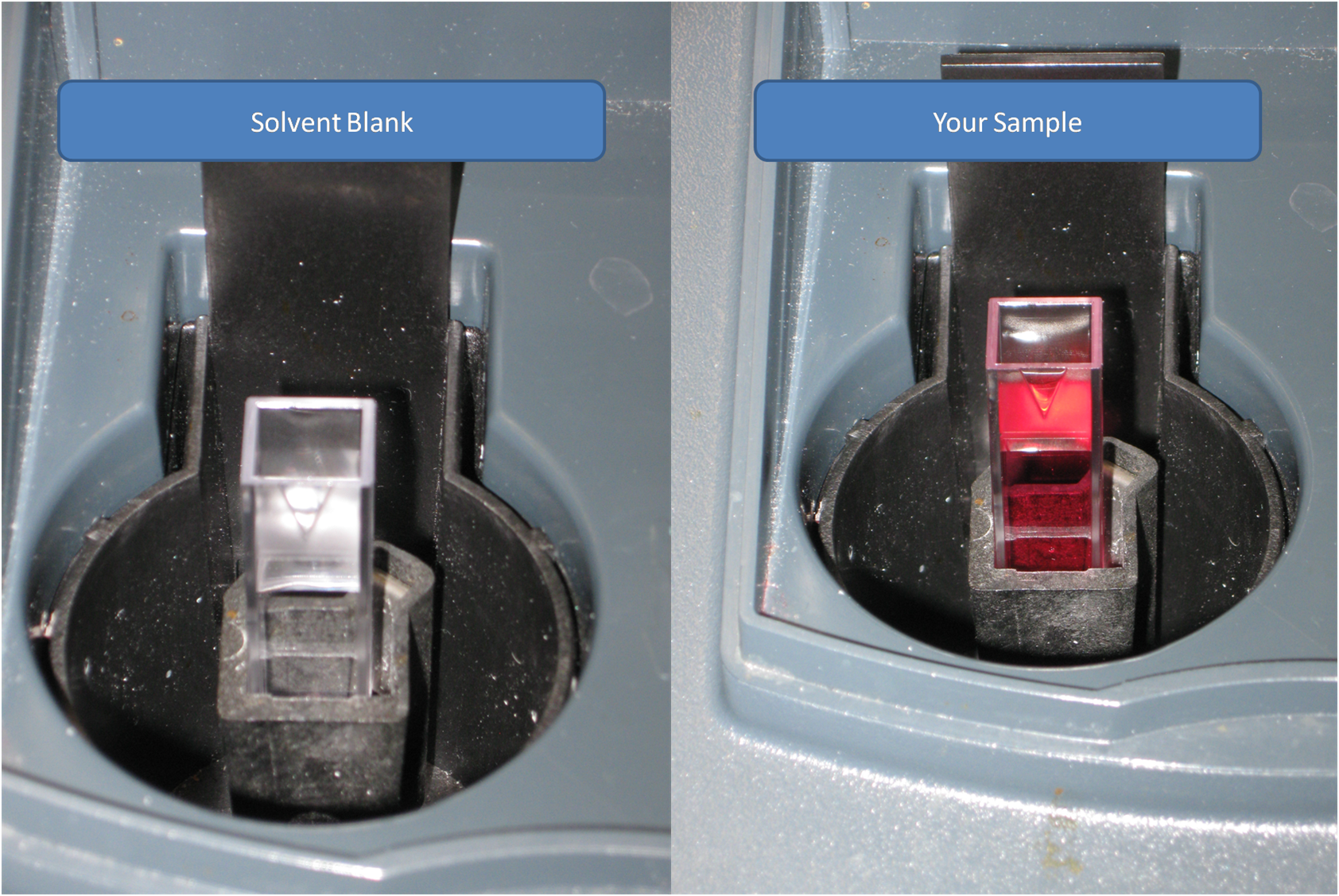
5). This procedure NEEDS to be repeated at EACH wavelength.
Absorbance is different for EVERY chemical at EVERY wavelength, so every time you change one of them, the spectrophotometer will need to be tared
Once you have your data all recorded, then you can plot each of your absorbance spectra
Y-axis will be the absorbance values
X-axis will be the wavelength
Look at the absorbance spectra that you create.
- Look at the maximum absorbance in your graph
- Look at the minimum absorbance in your graph
- What color are your solutions?
Show/hide comprehension question...
Experiment Goal
Generating and Using a Calibration Graph
Calibration Graphs are used to determine many things. In this experiment you are using a calibration graph to relate absorbance and concentration. They are used in many fields to relate a quantity with something you can physically measure. In the lab, you will prepare standards of a known concnetration, and plot a calibration graph. You will then use that graph to figure out the concentration of an unknown sample.
Terms you will need to know for the experiment
Dillution
Linear
Straight Line
Line of Best Fit
Absorbance
Concentration
Unknown
Concepts you will learn
What is a dillution factor?
What is a line of best fit?
What is a calibration graph and how do you use it?
Skills you will learn
How to dillute samples
How to generate and plot a calibration graph
How to work with the calibration graph to be able to determine an unknown
Generating and Using a Calibration Graph
Beer's Law
In the example of a calibration graph for this experiment, you are plotting absorbance vs. concentration, as opposed to an absorbance spectrum where you are plotting absorbance vs. wavelength. But how are wavelength and concentration related to absorbance? They are all related in through the Beer-Lambert Law.
The main absorbance equation is the Beer-Lambert Law which is:
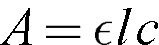
Where A is the absorbance
ε is the molar absorptivity constant. This is different for every chemical, and at every wavelength
l is the path length, the distance of solution that the light has to travel through
c is the concentration of the solution
The absorbance is based primarily on those three factors
Molar Absorptivity Constant
The Molar Absorptivity Constant is specific for every single solution, and at every wavelength. When you are taking an absorbance spectrum, and measuring the absorbance at different wavelengths, this is the only factor that is changing, as the concentration of the solution remains the same, and so does the pathlength. The path length of each vial is the same, and the concentration of each of these solutions is the same, yet the solutions are all different colors and will therefore absorb very differently. The only difference to change the absorbance, is the Molar Absorptivity Constant.

Questions to think about:
- Would the absorbance change if you use a different solvent?
- Which of the three factors would be affected by the change in solvent?
Path Length
The path length also affects absorbance. With a longer path length, the light has to travel through more solution, and can hit more molecules, and be absorbed. This would make the aborbance increase and have less light transmit through makeing the solution appear darker.
The pictures below show how solutions appear when you look through a longer pathlength.
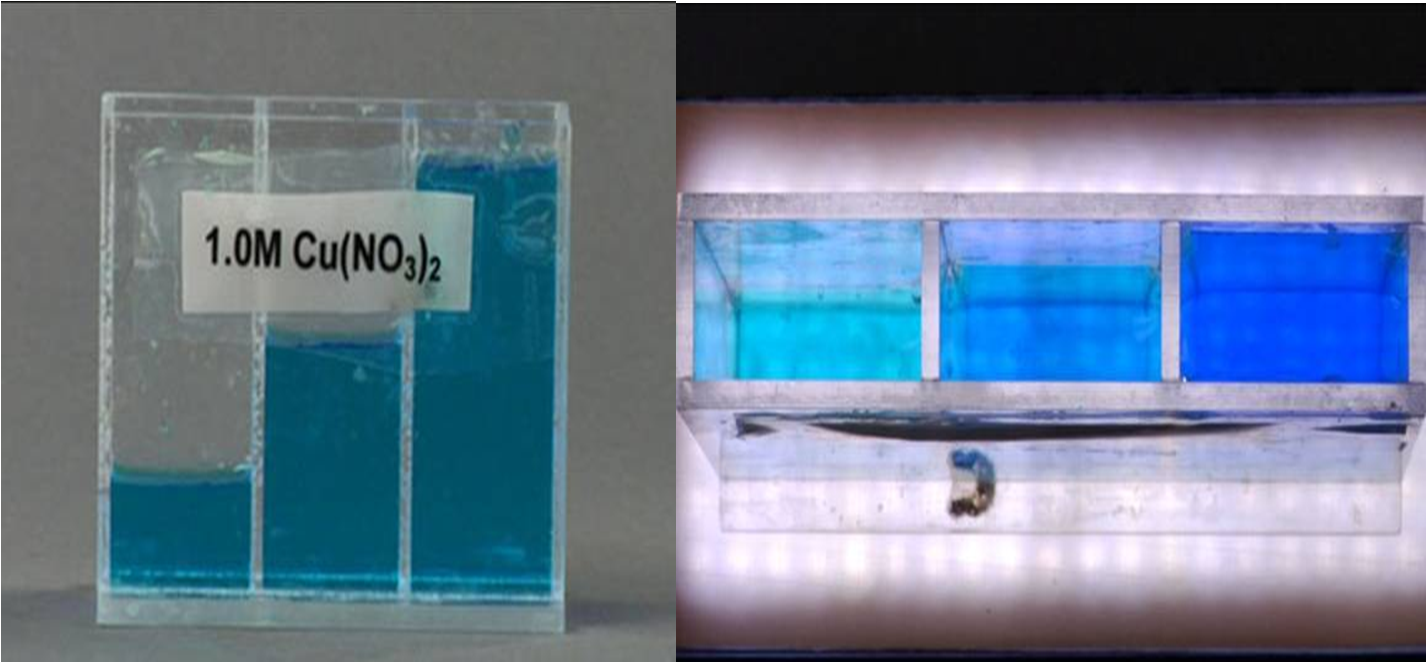
The image to the left shows a container with three compartments. Each compartment has the same solution but filled to different levels. When looking through the solutions horizontally, they all appear the same color because the path length is the same for all three. However, when you look at the image to the right, where the view is from the top, you can see that the solutions get darker as you move from left to right. The pathlength is increasing. There are more molecules in the way of the light, so more light can be absorbed and less will be transmitted to your eye.
This can also be seen in absorbance spectra, where as the pathlength is increased, the absorbance is also increased.
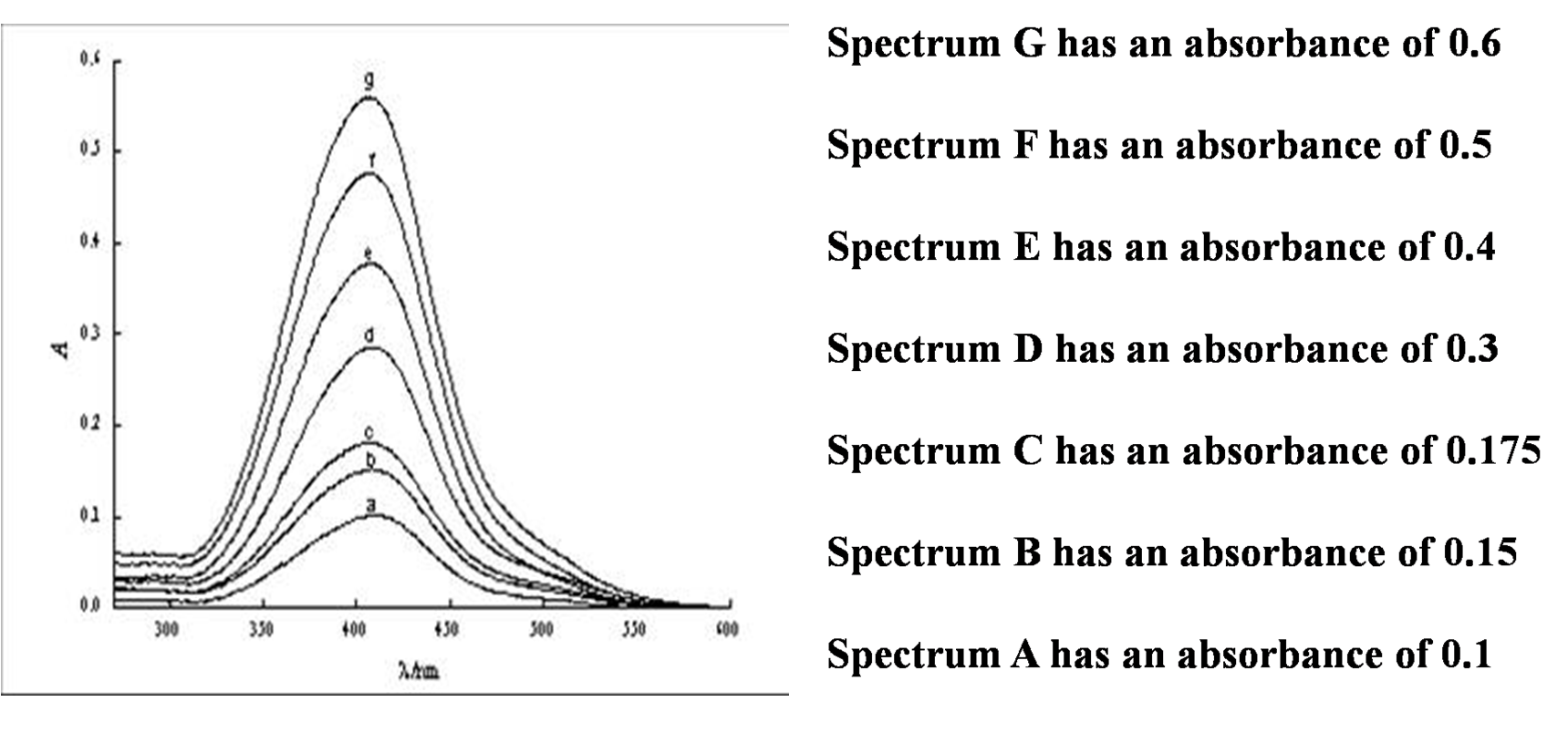
Using this information, and the spectra in the figure, see how the path length would effect the absorbance in the following questions.

Some important information is that path length is rarely ever changed away from 1.0 cm.
Concentration
The last component of Beer's Law, is concentration. Concentration effects the absorbance very similarly to path length. If the concentration of solution is increased, then there are more molecules for the light to hit when it passes through.
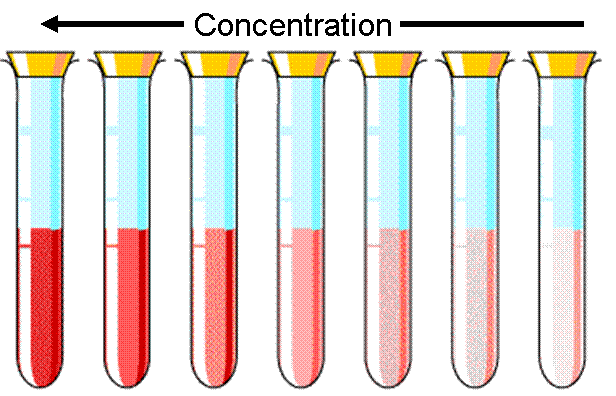
As the concentration increases, there are more molecules in the solution, and more light is blocked. This causes the solution to get darker because less light can get through.
Generating and Using a Calibration Graph
The Calibration Graph
When you take an absorbance spectrum, you are looking at the absorbance based on wavelength. But when making a calibration graph you are looking at the absorbance based on concentration.
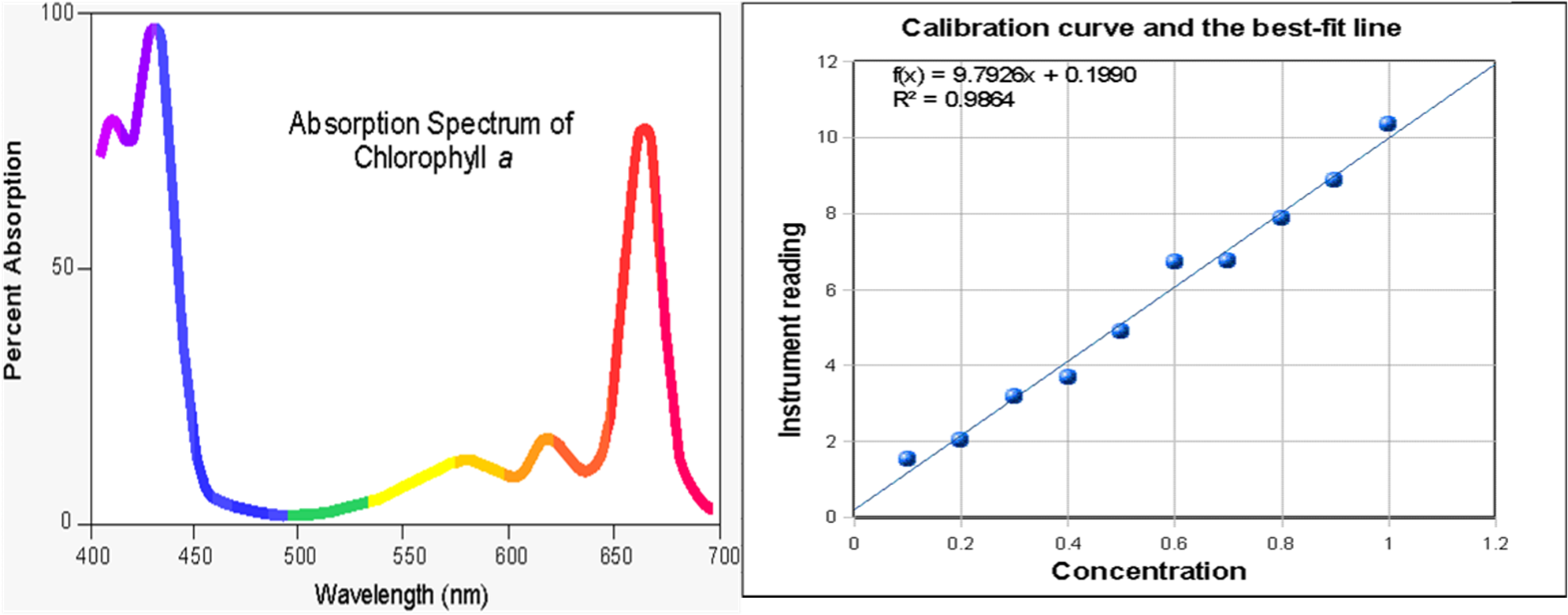
The two plots above are an Absorbance spectrum on the left, and a calibration plot on the left.
Both are plotting absorbance, but the spectrum plots it vs. wavelength (molar absorptivity constant) and the calibration plot is vs. concentration. When you take an absorbanc spectrum, the molar absorptivity constant is changing up and down and all around, but when you are working with a calibration plot, and are only changing the concentration, it shows a linear relationship between absorbance and concentration.
IMPORTANT NOTE!!!!!!
Beer's Law ONLY is linear at LOW concentrations!
If the absorbance of your sample is above 1.0 you will need to dillute your sample in order to lower the absorbance
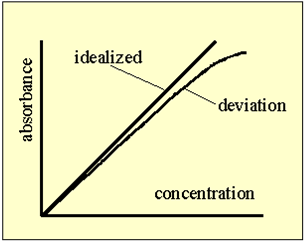
You cannot extrapolate your calibration line, because there is a deviation from a straight line at higher concentrations.
Having trouble with straight lines? Not to worry, here are some very helpfull sites to help you out! :)
- For information on "The Equation of a Straight Line"
- For information on "Best Fit Line"
- For information on "Scatter Plot with Fitted Regression Line (Excel)" 2003 Format
Generating and Using a Calibration Graph
Now You Try!
Now try opening a plotting progam and try making a calibration plot of your own. Please use the following values.
|
Absorbance |
Concentration |
|
0.68 |
1.0 |
|
0.61 |
0.9 |
|
0.54 |
0.8 |
|
0.46 |
0.7 |
|
0.39 |
0.6 |
|
0.32 |
0.5 |
|
0.27 |
0.4 |
|
0.21 |
0.3 |
|
0.15 |
0.2 |
|
0.08 |
0.1 |
|
0 |
0.0 |
The Wavelength used for this calibration graph was 410nm REMEBER THIS!!!
Make sure to include the following whenever you make your calibration graphs!
ALWAYS make sure they are labeled!
- Title (Include wavelength used)
- Axis
- Axis Titles
- Line of Best Fit
- Equation of the Line of Best Fit

Hint for plotting a line of best fit!
Make sure to FORCE your best fit line to go through the origin
Beer's Law has an intercept through the origin, so your best fit line should reflect that.
Another way to find the slope of a line, when you do not have a fitting program available, on a test for example, would be to calculate the slope. This would give you a less accurate slope, but would still be acceptable when a fitting program is not available.
IMPORTANT NOTE!!! On ANY Report or Exam, ALWAYS show your work when you preform any calculation!
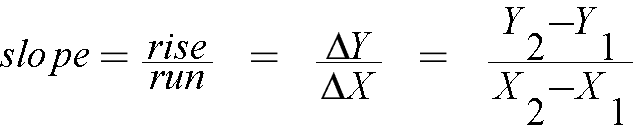
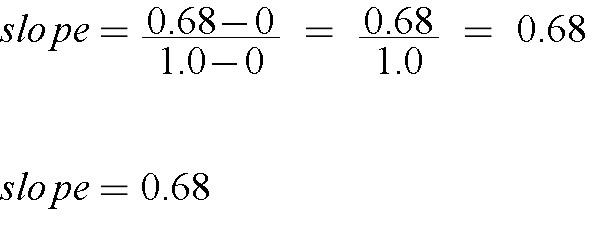
Generating and Using a Calibration Graph
How to Work with Plots
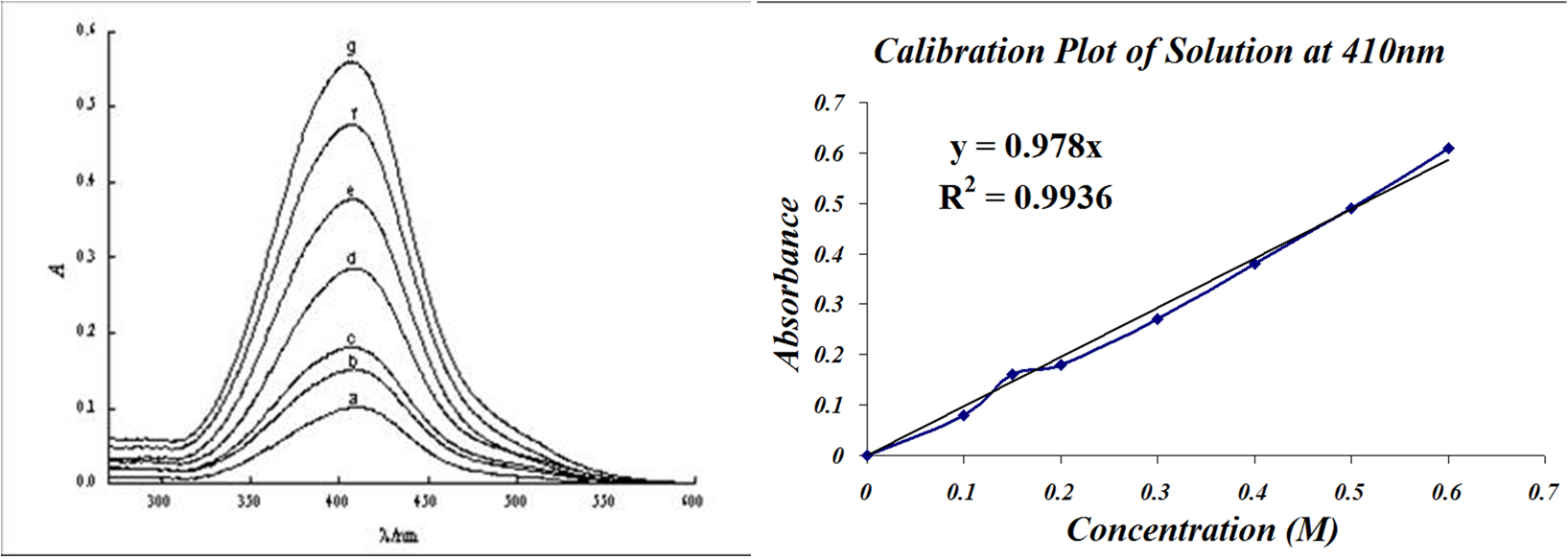
You have two different plots, your absorbance spectrum on the left, and your calibration plot on the right. The calibration plot is like taking a vertical slice through the all the absorbance spectra at the specific wavelength 410nm. The wavelength 410nm was a very good choice for the calibration plot, but how do you know which wavelengt is the best wavelength, based on the absorbance spectrum?
Choosing Your Wavelength
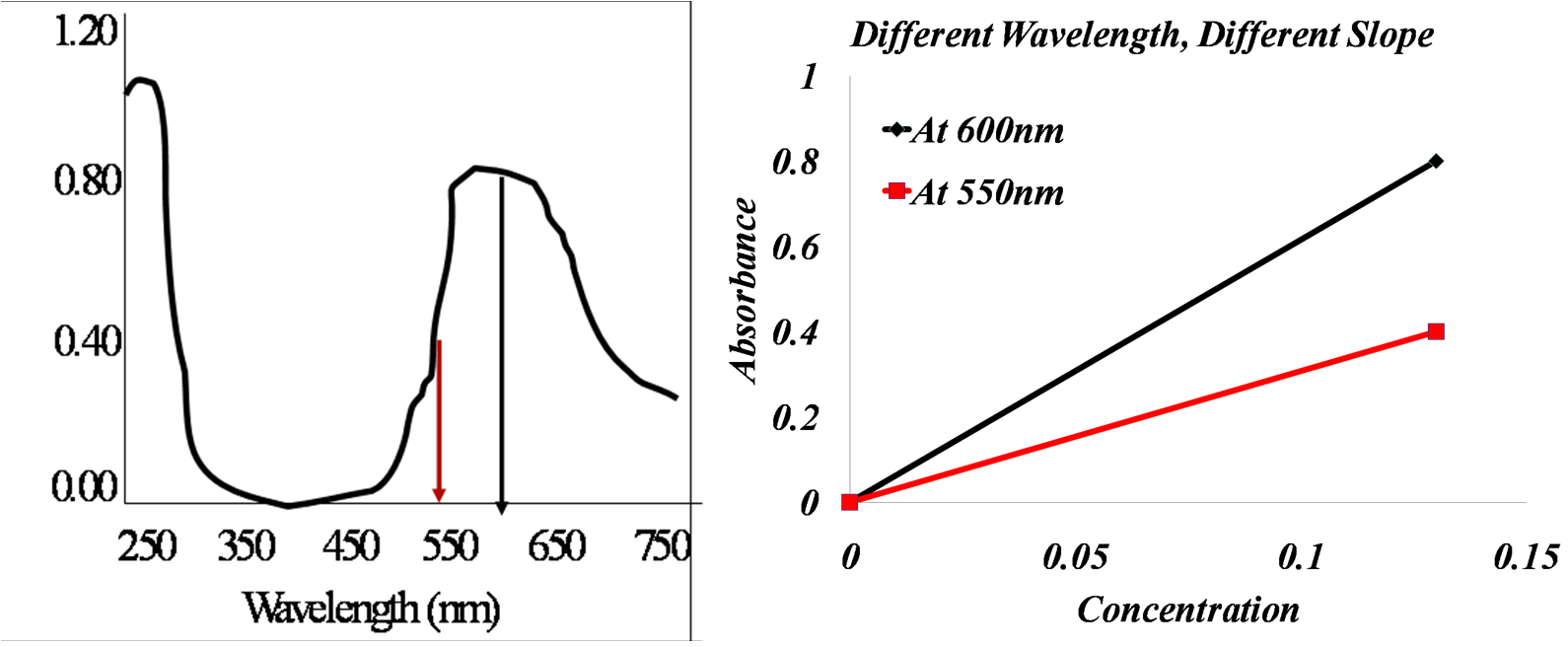
Look at the images above. The left is an absorbance spectrum of 0.13mM plastocyanin, while on the right is a calibration plot at two wavelengths.
Is the slope of the calibration line at 550nm greater than, less than, or equal to the slope at 600nm?
You can choose any wavelength to create a calibration plot, the only differerence will be the slope of the line.

When you actually choose your wavelength to create your calibration graph, you would generally like to choose a wavelength where there is room for the concentration to decrease. Look at the spectrum above. Do you think 450nm would be a good wavelength to use for a calibration graph? You would not choose that wavelength because when you lower the concentration, you would not be able to see much of a difference in the absorbance, and the calculations would be inaccurate. You would most likely want to choose wavelengths like 600nm or 250nm where there is a lot of room for absorbance change.
Generating and Using a Calibration Graph
Using your Calibration Graph!
Now for the fun part! Using the calibration plot that YOU made from the data two pages ago. We are going to determing the concentration of an unknown solution. Make sure you have your plot ready, because here we go!
Here's a typical problem. You take 3mL of your unknown sample and 7mL water and mix them together. The dilluted sample gives an absorbance of 0.432. What is the concentration of the initial unknown?
Where do you begin?! Well, you have your calibration graph, and it SHOULD look something like this, all properly labeled.
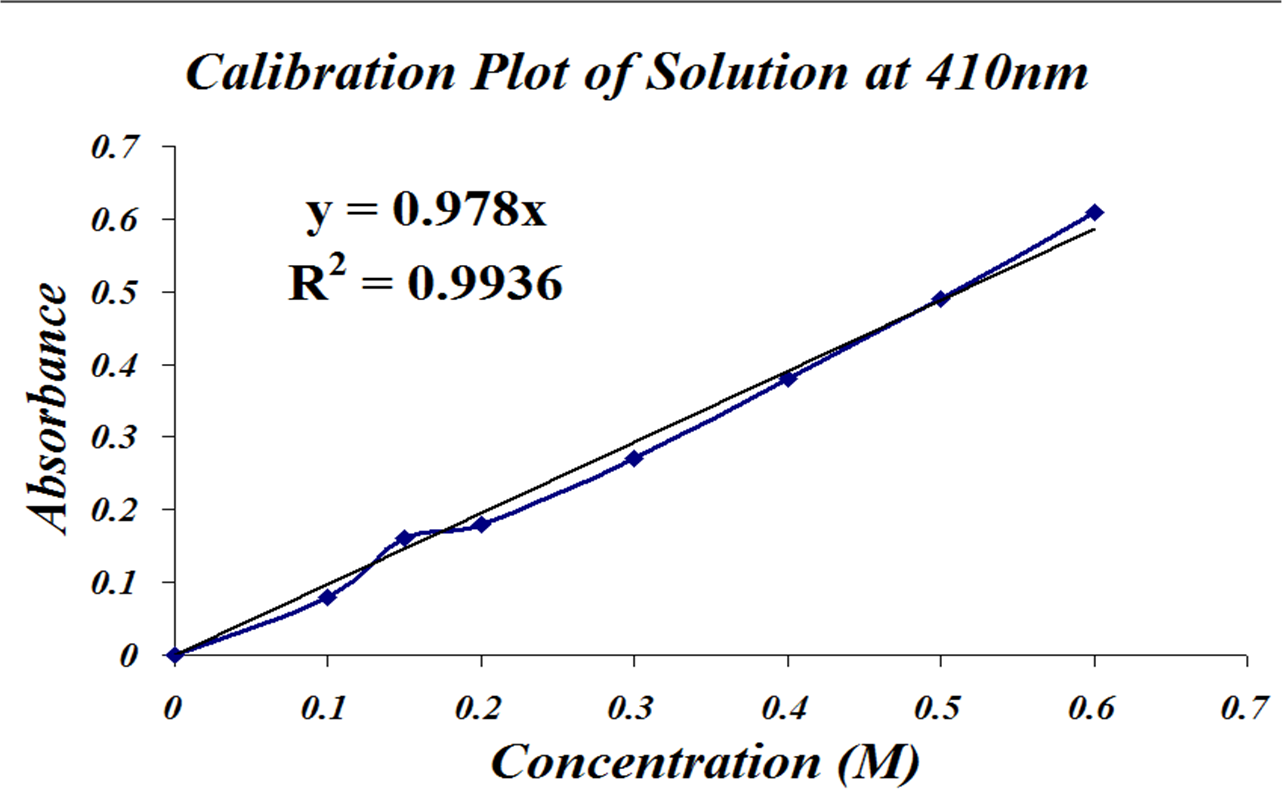
1). You have an absorbance, and you have a straight line equation that relates absorbance to concentration. This is the line of best fit through your data.
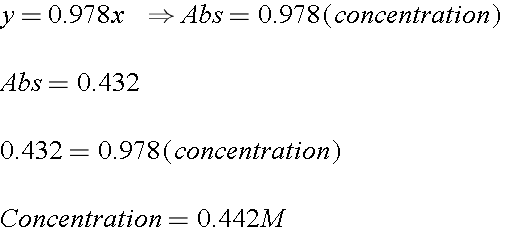

2). Now this is the absorbance of your DILUTED solution. But what was the concentration of your ORIGINAL solution? Remember you dilluted it once, so you can use the Dilution Equation
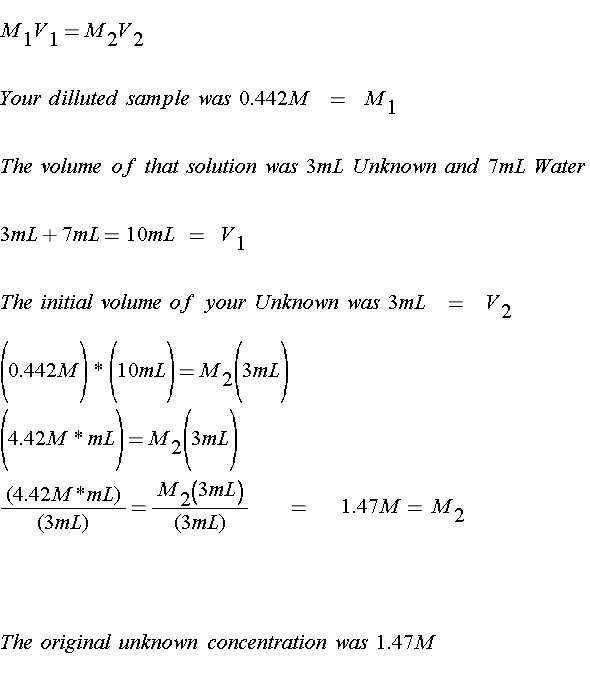
Ready to try one on your own?

Need some practice? Here are a few more problems. Try to figure them out on your own!

Common Errors In Calibration Plots
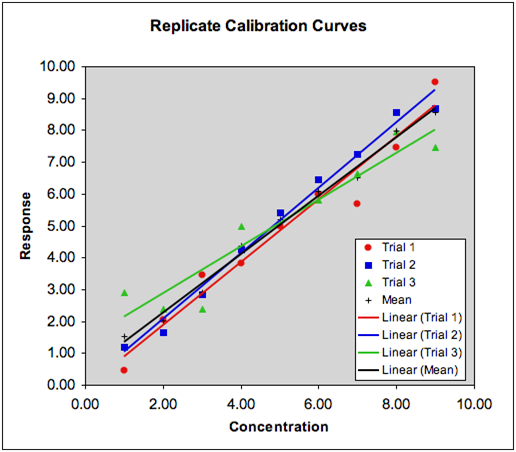
Final Thoughts
READ THE DIRECTIONS!!
You're Done! See you next experiment!
Questions?

For questions please attend the office hours of the GSI's or contact Nancy Kerner
nkerner(at)umich.edu