CHEM125
Precipitation and Water Purity
Introduction

 Learning Objectives for the experiment:
Learning Objectives for the experiment:
1. Design and interpret experiments to identify reactants and products in a precipitation reaction.
2. Determine if the physical properties of the reacting ions such as ion charge and/or size impact on its ability to stay in solution or precipitate.
3. Determine if the concentration of reactants impact on precipitation.
4. Determine why additional precipitation forms when some liquids other than water are added to water.
There are helpful hints, FYIs, and important facts included throughout the material. Place the cursor over the highlighted words for additional information. Scroll over bolded words within a sentence to view additional content.
Click on the quiz icon to toggle the questions on to make them visible.
Let's get started!
Required Background Knowledge and Skills
The types of compounds you will be using to collect data throughout the experiments are salts.
In order to perform satisfactorily in the lab you need to be familiar with certain knowledge, terminology, and background skills. For example, you will be working with salts throughout this experiment and thus you need to know what a salt is.
Below are action items you need to be able to do before starting the experiment:
- Describe what it means for a compound to be a salt
- Identify and correctly write formulas for salt compounds
- Describe the composition of salts
- Use the periodic table to determine the charge of ions in salt and write the formulas of salts given the ion charges
- Describe the terms solute, solvent, and solution
- Explain the terms polar and nonpoarl solvent
- Describe why salts are soluble and dissociate in polar solvents such as water
- Describe why salts are not soluble in nonpolar solvents
What is a salt?
Salts contain cations (positively charged ions) and anions (negatively charged ions).
- Metals lose one or more electrons to become cations.
- Non-metals gain one or more electrons to become anions.
Do you know what the difference is between an atom and an ion? The video below provides the answer to that question.
- As depicted in the video, copper (II) cation is formed from a copper atom when it loses its electrons to result an ion being positively charged.
Creation of a Salt
Salts may be created by the reaction of metals with nonmetals. The metal upon reacting loses electrons and the nonmetal gains electrons. Upon losing electrons the metal that is neutral in charge, converts to a positive ion (cation) and the nonmetal that is neutral in charge converts to a negative ion (anion). The video below depicts the reaction of sodium (Na) metal with the nonmetal chlorine (Cl2) to produce the salt NaCl containing sodium ions (Na+) and chlorine ions (Cl-). Note that during the process heat is released.
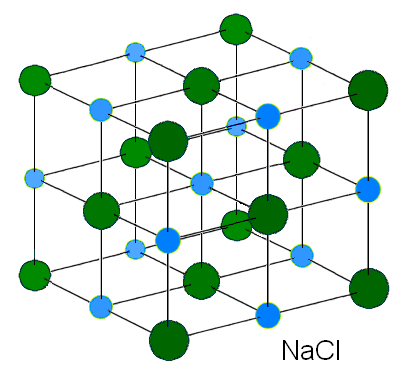
Once the ions, Na+ and Cl- are formed, they are stable and will not react with one another. The ions are arragned in a lattice structure where ions are surrounded by ions of the opposite charge (as depicted in the diagram on the left).
Salt Formulas
Could you correctly write a salt formula if you were told that the salt contained the ions Cr3+ and S2-? For an example of how to write such a formula, play the video below.
Key points to remember:
- The superscript charges on the cation/anion become the subscript for the anion/cation.
- Cr3+ and S2- = Cr2S3 where the salt itself has a net neutral charge. You need two Cr3+(totaling in a +6 charge) combined with three S2- (= -6 charge) to give a zero (net neutral) charged salt of Cr2S3.
- The superscript charges on the cation/anion become the subscript for anion/cation.
- If the subscripts for the cation and anion are the same in the salt formula,they "cancel" each other out.
- Ca2+ and O2-. Using the method described in the video, the salt formula could be written as Ca2O2. But since the subscripts are exactly the same, they "cancel" each other out and the correct formula of CaO arises!
- The subscripts should always be the simplest ratio!
- If any subscript is"1" it is not written in the formula.
- CaCl2 is not written Ca1Cl2.
IMPORTANT NOTE
Test yourself to make sure you know what you're doing!

Here's a quiz to see if you can identify the correct ions when given a salt formula!

Solvent Polarity
In order to understand WHY salts dissolve in water, we have to first understand solvent polarity.
The following video explains why water (a solvent) is polar.
- We learned that salt is made up of cations and anions.
- Due to the polar characteristic of water, this explains why salt dissolves!
- The partially negatively charged oxygen end (of water) is attracted to the cation and the partially positively charged hydrogen ends (of water) are attracted to the anion.
- They are then able to pull the cation and anion apart, causing dissociation.
Here is a demonstration of the attraction of water to a charge!
- Because of the static charge on the surface of the balloon, the partially charged (polar) water molecules are attracted, hence the bending of the stream of water towards the balloon.
- Since the hexane is non-polar, meaning it does not have an overall partial charge to it, there is nothing that is attracting it to the static charge present on the surface of the balloon. That is why you don't see the stream of hexane bending, like in the case with water.
Extra Challenge!
So what happens when a polar and non-polar solvent are mixed together?
Separate phases are formed due to polarity and density
-You deal with density is everyday life. For example: think about when you add ice cubes into a drink. The ice cube is less dense than the solution it's in, so that's why ice cubes float on top.
-You deal with different polarities of "solvents" when you eat! When you add an oil and vinegar dressing on your salad, you typically have shake it well before pouring to mix the two layers together. Why do you do that?
- It's because the dressing is in two layers (the two different polarities of the vinegar and oil causes them to be in separate layers). After you use the shaken dressing and left it sitting on the table, you will again see the two different layers forming.
- It will naturally form the two distinct layers because of polarity difference and the oil will always be on the top layer because it is less dense than the vinegar.
Take the quiz to predict what is occurring when you mix all three solutions together that have different polarities and densities.

The Relationship Between Salt Solubility and Solvent Polarity
How is this related to the actual salt dissolving?
- Salt is made up of ions (cations and anions) that carry each carry a positive and negative charge, respectively.
- Due to the charges of each ion, the water will therefore be attracted to the salt.
Here is a video that illustrates what is occurring when salt is dissolved into water.
Let's look at experimental proof that salt is indeed dissolving is water!
- Due to the ions (that were originally in the rigid lattice structure) are now free in the water- electricity can be conducted and that's why the light bulb shines.
So what happens when we change solvents from a polar solvent (water) to a non-polar solvent such as hexane?
Make an hypothesis
Let's look at it experimentally!!
- The salt remains in its original rigid lattice structure (that's why you see the "undissolved" salt at the bottom of the beaker).
- Because the salt is still solid, there are no free floating ions in the solution to allow for electricity conduction.
The Relationship Between Salt Dissociation and Solvent Polarity
So what is actually occurring what salt is dissociated into its ions? Why does this occur?
Precipitation Reaction
What you need to understand here, is that precipitation and salt solubility go hand in hand but they are NOT the same concept.
During this experiment,precipitation will be referring to when a new salt complex is formed and comes out of solution.
- When the initial salt does not dissolve in the solvent- it is not thought of as a precipitate.
- As seen in the reaction in the video, the initial salts, KI (aq) and HgCl2 (aq) are soluble in water, meaning they completely dissolved when you add each respective salt by itself into water.
- Remember, when the salt dissolved- for example KI, the K+ and I- ions are no longer in the rigid lattice form but are separated from each other by being surrounded by water. Hence you have "free" floating ions in solution.
- Therefore, if you added the two salts together- the free floating ions react to one another, and in this case, some of the Hg+ ions reacted with the I- ions to create a salt of HgI that precipitated out (becoming the precipitate). K+ ions can react with either Cl- or I- in solution (both products are soluble!)
- Not all the Hg+ ions will react with I- ions. You would have HgCl2 also in solution mixture, but since it is solule- it will not precipitate out.
One important key note to remember is that, even if you just are dissolving one salt, you can get that salt to precipitate out of solution if you exceed the ion concentration threshold.
Here is an example to explain the concept of precipitation when the salt concentration exceeds the saturation level.
- The sodium acetate hydrated salt has a solubility value of 50.4g/100g H2O.
- Meaning, you can dissolve 50.4g of the salt in 100g of water and have all the salt dissolve. If you dissolve perfectly 50.4g, the solution is saturated.
- As seen in the video, if you add even a grain of salt to the saturated solution, it exceeds the saturation level (ionic concentration threshold) and you see the salt precipitate out.
- Remember: this is a precipitation because the initial salt dissolved in water. If it didn't dissolve in water in the first place, the salt sitting on the bottom of the flask would not be considered the precipitate.
Summary of key points to fully understand what is going on in lab.
- salt consists of an anion (negatively charged) and a cation (positively) charged.
- Salt dissolving depends on the polarity of the solvent
- a polar solvent, such as water, has partial charges on the molecule (that's why it is polar).
- the partially negative charge from the oxygen will attract to the cation
- the partially positive chage from the hydrogen will attract to the anion.
- because of the partial charges, the water will form a shell (see diagram above) around the ions (essentially separating them apart- which is what dissolving is all about).
- a non-polar solvent, such as hexane, does not have any overall partia charge to it. So there is nothing attracting the ions of the salt (so it will remain a solid and will not dissolve).
- You have to distinguish the difference between precipitation and salt solubility. The concepts work together but they are not the same thing!
- if the salt is not soluble in a solvent (not dissolved)- the remaining undissolved salt is not the precipitate.
- precipitation (in this experiment's case) is thought as: when two different ions combine together and create a new salt (different than that of the original salts used).
- Simply put: if you have salt AB and salt CD, both have to dissolve in the solvent (hence getting free floating A+, B-, C+ and D- ions. The ions can then interact and create new salts:AC and BD (and also combine to make the original salts).
- If salt AD has a low solubility- the water can "retain" a very small amount before the ionic conctration is over the solubility threshold and you start seeing the salt precipitating out.
- If salt BC has a higher solubility- the water can "retain" a larger amount. It would take a higher concentration to go over the solubility threshold for the salt to start precipitating out.
Now let's move onto learning about the experiments being done in lab!
Experimental Determination of Precipitate Identity
During lab you will investigate precipitate reactions and identify the products or precipitation reactions. A question you will address is "What is the precipitate?"
In order to appropriately answer such a question you need to know how to:
- Use the CRC Handbook to look up the properties of substances.
- Design reference blank tests to experimentally identify the spectator and reactant species in a reaction.
The CRC Handbook of Chemistry and Physics is the world's most popular scientific reference book. It features tables and reference sections on everything including many properties of chemicals such as solubility, color, melting point.
Below is a video guide on how to look up data on chemicals. The video below demonstrates how to look up the solubility of a salt.
- Starting from the University of Michigan home page:
- Click on the Mirlyn catalog link under quick links.
- Click on Mlibrary home.
- Type in CRC handbook in search bar (have only the database option selected).
- From the results, select the CRC handbook of Chemistry and Physics.
- Expand Section 4: Properties of the Elements and Inorganic Compounds
- Select Physical Constants of Inorganic Compounds.
- There are many abbreviations used in the text, take a moment to read over and familiarize yourself with the terms.
- Type in the salt name you wish to search in the "find" box.
Reference Blank Test
- An experimental test mixture that is designed to identify reactants and specators (non-reactants) is called a reference blank test. A reference blank test:
- Omits a species from the reaction.
- Substitutes a known spectator species for the omitted species.
The video below describes an analysis of the precipitation reaction between HgCl2(aq) and KI (aq) using reference blank tests. A reference blank test is demonstrated and the conclusion is stated. Watch carefully as the second reference blank test is demonstrated. You will be asked to answer the following question at the conclusion of the test demonstration: What do you know about the tested species in the precipitation reaction based on the outcome of the second reference blank test?
Test Yourself.
What do you know about the precipitation reaction based on the outcome of the reference blank test where NO3- was substituted for I-?

If you wish to further check your answer and reasoning watch the video below. Also observe the outcome of a second reference blank test testing another ion!
In the second reference blank test done in the video, what conclusion can be made based on the outcome where NO3- was substituted for Cl-? Take the quiz!

Reference blanks tests are an experimental method used to identify reactants and spectators in any type of reaction.
Based strictly on the above tests can you indicate that Hg2+ is a reactant in the precipitation reaction?
F.Y.I.
You need to keep some important things in mind when creating a reference blank test!
- Just because you made substitutions of ions, doesn't necessary mean your reference blank test is valid.
- Always remember: a reference blank test results either gives you a reaction that gives you the exact same product or there is no reaction in order for it to be a valid test that you can use to make conclusions about reactants and spectators.
Let's look at an exam question that has to do with reference blank tests.
Experimental Analysis of Precipitation Data of Different Metal Ions
Investigate possible links between precipitation and ion characteristics.
- You will look at different reactions in order to determine if precipitation is predictable based on the periodic table.
- It is the objective to determine if a trend emerges as you look at different cations that are in the same family as well as the same period.
- You will have to take into account what kind of properties (ion size, charge, etc) are seen in the periodic table among the different ions and associate that with how one ion would more likely to precipitate out compared to another.
Experimental Determination of the Impact of Concentration on Precipitation
Determine if some minimum concentration is required for precipitation
- Here you will look at the relationship between salt solubility (the concentration of ions) and precipitation
- So it all depends on the concentration of the ions inside the solution, whether or not you will observe the precipitation of the salt in solution.
- Remember: the ion concentration has to be over the solubility threshold in order to precipitate. Just because you don't see precipitate it doesn't mean there aren't ions in the solution.
So here's a question to ponder about: do all the reactants in a reaction react together to form a precipitate?
Let's test to see what ions are leftover.
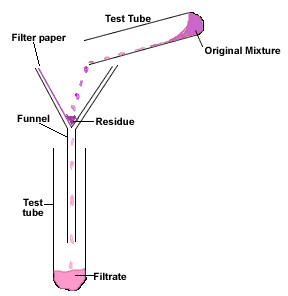
- The video demonstrates the process of filtration, which allows to determine whether all the reactants are used up to produce the products.
- The filtration system is initially set up by first placing the rubber adapter onto the glass funnel and then placed into an Erlenmeyer flask.
- The circular filter paper is folded in half and then folded in half again (so the filter now quartered and looks like a cone).
- The filter paper is placed in the glass funnel and rinsed with DI water to stick the filter paper onto the walls of the funnel.
- KI (aq) and HgCl2 are reacted together in a separate test tube, creating a pink precipitate.
- The original mixture is poured into the filter paper, the precipitate being caught in the filter and the solutiom drips out into the flask.
- KI (aq) is added to the filtrate (solution in the flask).
- As proven in the demostration, even though the reaction of: KI (aq) + HgCl2(aq) initially produced a precipitate, upon the addition of KI(aq) to the filtrate in the flask produced more precipitate.
- This means that all the reactants didn't combine together to produce the pink precipitate and there were some left over to react with more KI(aq)!
Experimental Determination of the Impact of Different Solvents on Precipitation
Compare the impact of water and solvents other than water on water purity and precipitation
- Here you will be experimenting with different solvents (different polarties) and observe the effect of solvent polarity has on salt dissolving and precipitation formation.
Hypothesize what would happen if you add acetone to saturated CuSO4(aq)
Let's look at what happens when acetone is added to the saturated Copper(II) sulfate.
- The less polar solvent, acetone, is added to the most polar solvent, water (copper (II) sulfate solution).
- Acetone will mix with the water due to the fact that acetone is moderately charged (so there will not be two separate layers of solution).
- As the acetone is mixed with the copper(II) sulfate, it decreases the polarity of the overall solution.
- The salt (copper(II) sulfate) solubility decreases, causing the blue salt to precipitate out.
Remember:
- Water is the most polar solvent; when salt is dissolved in water, the ions are separated and protected by the water shell.
- Other moderately polar solvents, such as acetone, will mix with the water and decrease the polarity.
- Non-polar solvents, such as hexanes, will not mix with water and two distinct layers will be observed.
- Salt solubility (how much you can dissolve) will change as the solvent or its polarity is changed.
Let's look at some questions that sums up what you just learned from this lession

Now that you have the basics, you are ready to fully understand what is going on in experiment 1 in lab!!
Optional Reviews and Extensions
- First you need to familiarize yourself with the periodic table. It's the fundamental knowledge that is acting as a base for everything you will be coming across in Chem125/126.
- You're not expected to memorize the common ion charges, but you want to understand the information provided in the periodic table. Get familiar with the table so you're not seeing it for the first time on the test and have no clue what it means.
- Note that the family number (1A, 2A, etc) is the same as the maximum positive charge for the element that resides in that family.
- The number of available electrons for reaction (as metals) are the same as the family number.
F.Y.I
- 3A-6A groups: have two oxidation numbers other than zero.
- the electron in the outer energy levels are filling two different sub-energy levels.
- the post transition metals: 2 electrons in the s sub-energy levels and varying number of electrons in the p sub-level.
- typically the electrons in the s sub-energy levels are lost first, that's why there is a difference of two between losing all and losing some of the electons.
Helpful Hint


![]() Learning Objectives for the experiment:
Learning Objectives for the experiment:






