Diels-Alder Cycloaddition Reaction
Presentation given by: Angela Liang, Roberto Morales, and Sara Scott
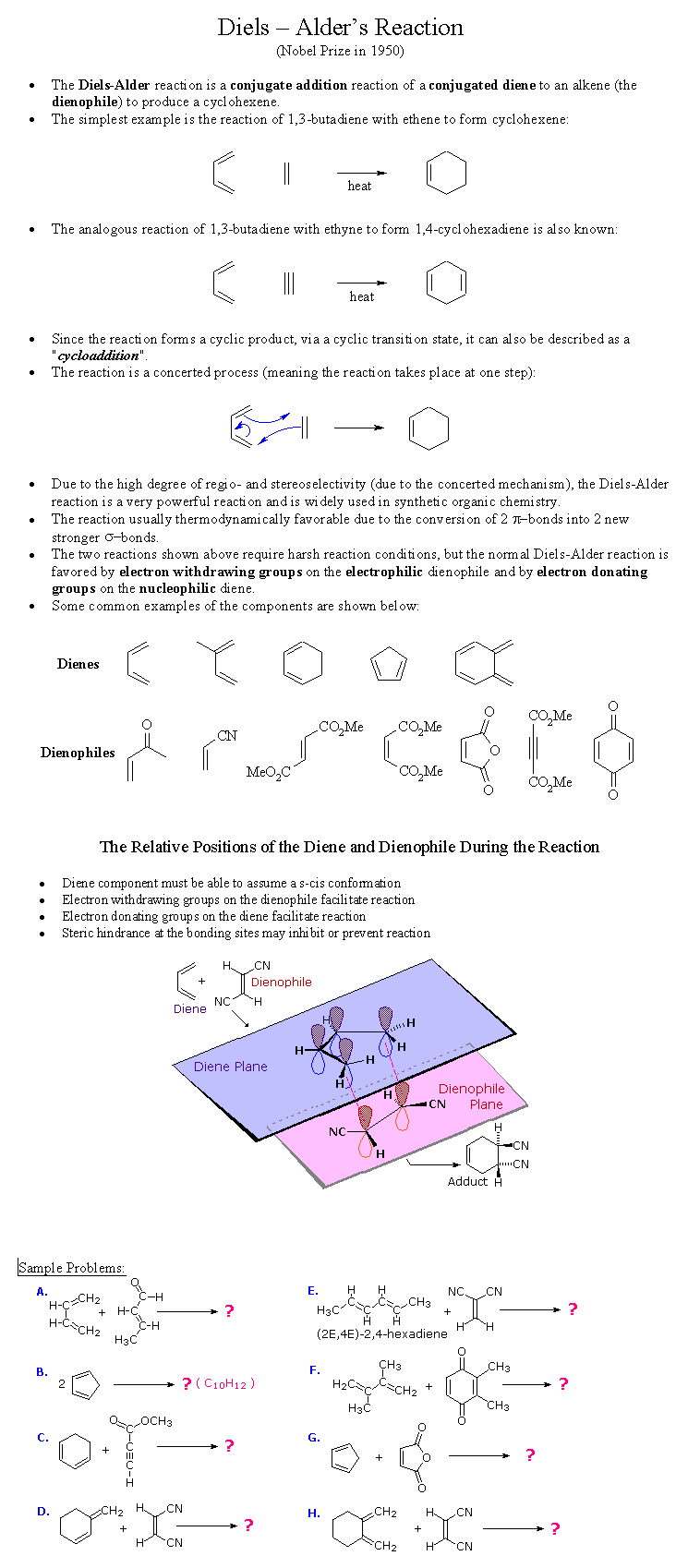
The Diels-Alder Rxn is a very simple, but elegant reaction. It
translates stereochemical information through the reaction. This is
stereospecificity.
However sometimes one of those products is more favored for one reason or
another. This is stereoselectivity of one product over another. And
finally the Diels-Alder Reaction has regioselectivity. This means the
molecule can choose its 3-D orientation to react in. See Below for
examples, more explainations, and practice problems.
Click here to see an
example of Stereoselectivity in a Diels-Alder Reaction
Click here to see an
example of Stereospecificity in a Diels-Alder Reaction
Click here to see an
example of Regioselectivity in a Diels-Alder Reaction
Click here to see the
stereochemical relationship Endo/Exo in bicyclic
compounds
Click here
to see a great ANIMATION of the Diels-Alder Rxn
Here's
another to check out
Check the answers
here
