Leading Question: How general is this olefination reaction- find and present other examples of the reaction.
Wittig Reaction:
The Wittig Reaction allows the preparation of an alkene by the reaction of an aldehyde or ketone with an ylide generated from a phosphonium salt. An ylide is a neutral molecule with positive and negative charges on adjacent atoms. Most often the ylides used in Wittig reactions consists of a phosphorus atoms bonded to three substituents and a carbon bearing a negative charge. The stereochemistry of the molecule depends on the reactivity of the ylide. If the substituents on the phosphorus are phenyl groups, then the ylide is stabilized and gives mainly E configuration. If the substituents are alky groups, the ylide is more reactive and gives Z configuration.
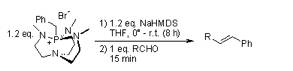
Wang, Z.; Zhang, G.; Guzei, I.; Verkade, J. G. J. Org. Chem. 2001, 66, 3521-3524.
Horner-Wadsworth- Emmons Reaction:
The Horner-Wadsworth-Emmons Reaction, also known as the Wittig-Horner Reaction, is similar to the Wittig Reaction. It uses aldehydes or ketones with stabilized phosphorus ylides to form olefins with favored E-selectivity.
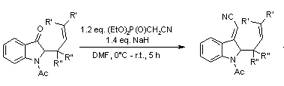
Kawasaki, T.; Nonaka, Y.; Watanabe, K.; Ogawa, A.; Higuichi, K.; Terashima, R.; Masuda, K.; Sakamoto, M. J. Org. Chem. 2001, 66, 1200-1204.
Peterson Olefination:
The Peterson Reaction forms alkenes from a- silylcarbanions and aldehydes or ketones. The intermediate b- hydroxysilane may be isolated and treated with either base or acid to give different stereochemistry outcome. After the addition of the silylcarbanion to a carbonyl compound, the reaction forms diastereomeric adducts. Acidic hydrolysis of the intermediate leads to Z-selectivity while the base-catalyzed elimination leaders to E- selectivity.
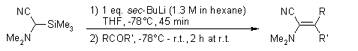
Adam, W.; Ortega-Schlute, M. Synlett 2003, 414-416.
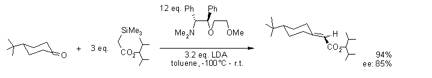
Iguchi, M.; Tomioka, K, Org. Lett. 2002, 4, 4329-4331.
Julia Olefination:
The Julia Olefination or the Julia-Lythgoe Olefination is a multistep synthesis that enables the preparation of E-selective alkenes. The reaction utilizes a phenylsulfonyl carbanion to add to an aldehyde or ketone. The intermediate alcohol is esterified in situ and then reductive elimination occurs with sodium amalgam to furnish the alkene.
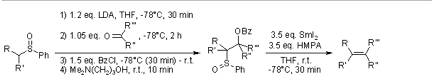
Pospisil, J.; Pospisil, T.; Marko, I. E. Org. Lett. 2005, 7, 2373-2376.