Dess- Martin Periodinane Reaction Tutorial
The Dess-Martin periodinane oxidation reaction has proven to be a successful and effective synthetic tool to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. It has high selectivity for hydroxyl groups in complex alcohols, and will not further oxidize aldehydes to carboxylic acid. It has several advantages over chromium oxidizing agents, such as the Jones oxidation or Swern oxidation, because it is safer, milder, quicker, and involves simple work-up procedures for purification of the final product.

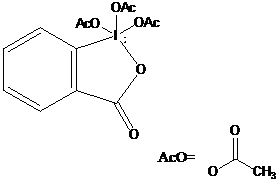
The compound “Dess-Martin periodinane” is shown here. It’s chemical name is 1,1,1-Tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3(1H)-one. It is available through SigmaAldrich either pure or in a solution of dichloromethane. It is expensive however, and a procedure for synthesis was outlined in the original article by Dess and Martin.
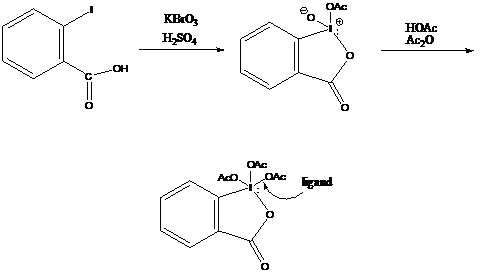
Treatment of 2-iodobenzoic acid with KBrO3 in sulfuric acid gives the cyclic tautomer of 2-iodoxybenzoic acid in 93% yield. When this acid is treated with acetic anhydride and acetic acid, and heated to 100° C gives the Periodinane in 87% yield. The periodinane has an indefinite shelf-life when kept in a sealed container.
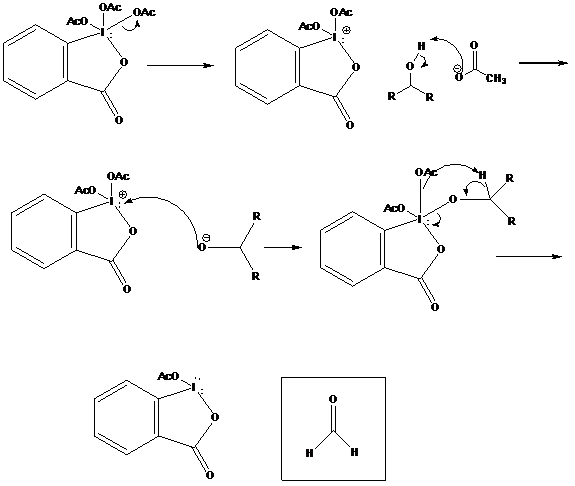
The mechanism for oxidation involves the exchange of acetoxy ligands for alkoxy ligands. Ligand is a chemical term for a substituent that donates its electrons through a covalent bond to a central atom, in this case iodine. The periodinane is dissolved in either chloroform or dichloromethane, and the reaction is carried out at room temperature.
The reaction begins with one of the acetoxy groups from the periodinane leaving. It deprotonates the alcohol to form acetic acid. The negatively charged oxygen from the alcohol attacks the now positively charged iodine. An intermediate iodine-ester complex is formed. The next step is a series of electron transfers. One of the attached acetyl groups leaves and deprotonates the ester. The electrons from the carbon-hydrogen bond shift to the oxygen, creating a carbonyl. The electrons from the iodine-oxygen bond are transferred onto the iodine as lone pairs. The two resulting species are the oxidized alcohol, now a ketone, and a neutral iodine species with two lone pairs.
This reaction is clean and simple. By-products are easily removed by filtration or chromatography. Further research has been done by the original authors proving that other halide compounds similar in structure can serve the same function. The procedure is commonly used for various synthetic applications.
For further information, the citations for the original article and follow up research by Dess and Martin have been provided along with as a few helpful websites in the references section