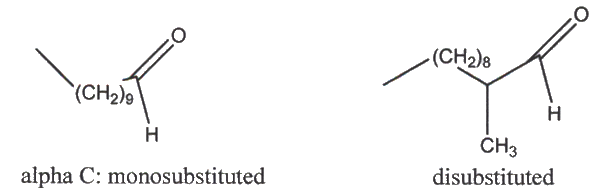|
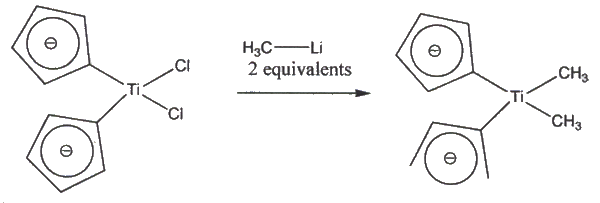
III. Experimental: (30): T a solution of 28 (9 mg, 0.03 mol) in toluene, was added a stoichiometric amount of MgBr2, and the mixture was stirred at room temperature for 15 hours. Water was then added to quench the reaction. The aqueous layer was extracted 3 times with diethyl ether (3 x 10mL). The organic layer was dried over MgSO4 and filtered, and the solvent was evaporated. The crude product was purified by flash silica chromatography (10% EtOAc/hexane) to afford 11 mg of 30 (99% yield) as a colorless oil. IV. Related Papers to Step 27 Titanium-Mediated Carbonyl Olefinations. I. Methylenations of Carbonyl Compounds with Dimethyltitanocene Petasis, N.A.; Bzowej, E.I. J. Am. Chem. Soc.1990, 112, 6392. The Petasis Reagent is a more practical reagent to use in the methylatio of an aldehyde, ketone, lactone, or ester, as it is free from synthetic difficulties. For instance, it is not possible to convert an ester or lactone using Tebbe or Wittig-type reagents. Also, the Petasis Reagent, Cp2Ti(CH3)2, is readily prepared from titanocene dichloride, as shown below.
Olefinations required at least two equivalents of Tebbe's reagent drives the reaction to completion. Teh icnreased reaction rate in THF suggests polare intermediates. Research shows that readily enolizable aldehydes and ketone were efficiently olefinated. Also, a disubstituted alpha carbon in an aldehyde reacts faster than a mono-substituted alpha carbon.
Esters and lactone reacted at a slower rate, due to different conformational geometry of the titanium intermediate complexes.
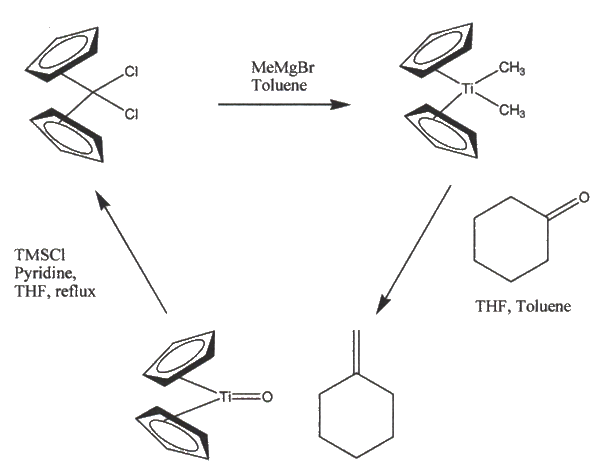
Additionally, in esters,a different intermediate may be formed that allows observed H/D scrambling to take place between the ester and Cp2Ti(CD3)2 Other Papers Related to Step 27: 1. Berget,P.E.; Schore, N.E. Organometallics. 2005, 25, 552-553. a. Shows how to recylce Titanochene Dichloride using the Petasis Olfefination.
2. Cook, M.J.; Fleming, E.I. Tetrahedron Lett. 2005, 46, 297-300. a. Use of microwaves increases reaction rate with petasis Olefination. 3. Mesaros, E.F.; Meyer, E.A.; Smith III, A.B. J. Am. Chem. Soc. 2005, 127, 6948-6949. a. Used in Petasis Olefination in order to accomplish a rearrangement and ring closure in synthesis |