





LEADING QUESTION
Why is the mechanism for 2 (R=H) to 5 (R=H) important to prepare the natural product vinigrol?
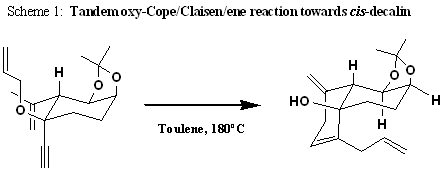
The vinigrol structure reveals a cis-decalin connected to the eight-membered ring (see Figure 1). The cis-decalin could be realized in the tandem oxy-Cope/Claisen/ene reaction of the allyl ether 2. In Figure 2 below, the rationalization for the diastereoselectivity is explained in the reaction mechanism proposed by Morency et. al.
Figure 1. The structure of vinigrol.
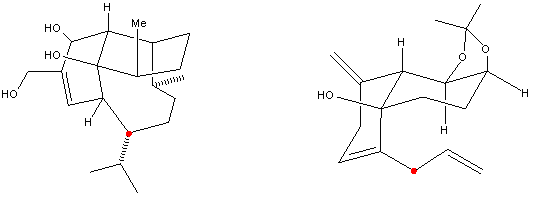
Overall allyl ether 2 undergoes three intramolecular rearrangements. The first rearrangement is an oxy-Cope rearrangement. Two transition states A and B are possible. A is a more thermodynamically stable transition than B because the substituents on the chair conformation are sterically hindered by 1,3-diaxial interactions. In A the ether substituent is in the equatorial position which reduces such an unfavorable steric interaction.
The macrocyclic allene has two carbon-carbon double bonds formed as a result from the oxy-Cope rearrangement. The strain from such a conformation leads to the second rearrangement. The Claisen [3,3]-shift forms a ketone in 10-membered ring system.The third rearrangement is a transannular carbonyl-ene reaction where the electrons are cyclized in six-membered ring via two possible transition states C and D. C is favored over D in the transition state because D has 1,3-diaxial interactions between its substituents in the chair conformation.
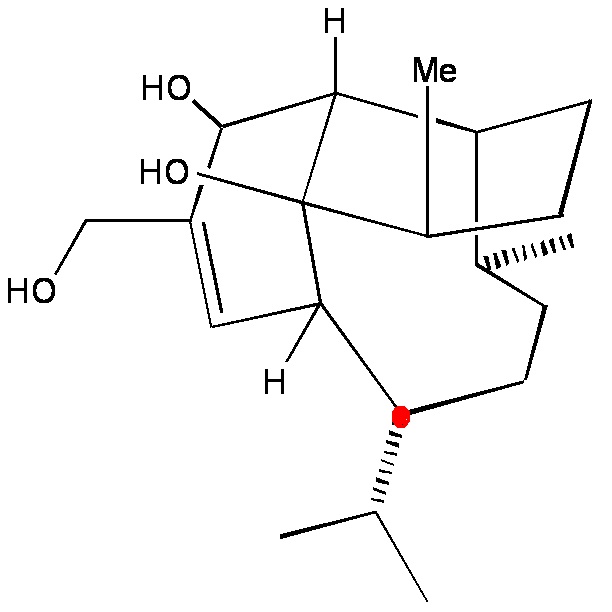
The vinigrol moleculehas an iso-propyl group. In molecule 5, the iso-propyl group would have to be added at C9 for further synthesis of vinigrol. However, when a larger substituent is in place of the hydrogen at the terminal allylic position in 2 (see Figure 3), Morency et al. observed that the larger substituent is a hindrance to the cascading intramolecular rearrangements in the mechanism from 2 to 5. Thus, a substituent in place of hydrogen would make it more difficult for later addition of the iso-propyl group.
Figure 3. Comparison of vinigrol and cis-decalin, which is the product under investigation in this manuscript project by SSG 5-7.
vinigrol with the iso-propyl group at C9 and cis-decalin with no substituent at C9
Figure 2. Mechanism for molecule 2 to 5.

Morency, L.; Barriault, L. Tetrahedron Lett. 2004, 45, 6105-6107.


