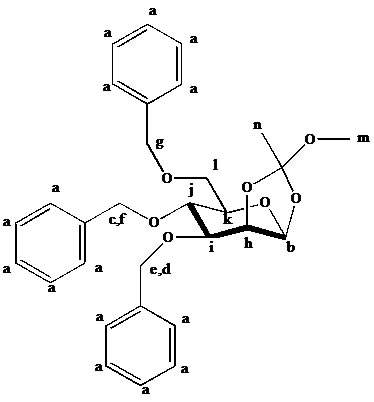
Explanation of Assignment of H NMR Peaks for Molecule 25
d7.41-7.43: m, the amount of hydrogen atoms (15) as well as the chemical shifts are clearly indicative of three mono-substituted aromatic rings.
5.10, 4.11: b, is highly effected by induction due to the two adjacent oxygen atoms, and shows a small axial / equatorial coupling constant. The doublet corresponds to h, which is shifted less downfield due to fewer inductive effects, but still exhibits a very small axial / equatorial coupling constant.
4.92, 4.63: c and f, which have identical coupling constants. They also have high shift values as they are the middle hydrogen atoms in an –OCH2PH group, flanked by to identical structures, and are effected more by induction through the sigma bond structure.
4.83, 4.76: d and e, which have identical coupling constants. They also have high shift values as they are the middle hydrogen atoms in an –OCH2PH group, but lower than those of c and f as they are not flanked by two identical structures.
4.53: g, which has a higher shift than the ring bound hydrogen atoms, but lower than the other benzyl H’s as it is more removed from the ring.
4.02: i, which has a high value than j based on inductive effects and exhibits a doublet of doublets with Hh and Hj
3.76: j, the similar coupling constants for the two doublets signify very similar neighbors. These correspond to Hi and Hk which are both also in axial positions.
3.76-3.71: the 4H likely correspond to k and l, which have complex splitting patterns due to the sheer amount of chemically non-equivalent H’s. These are constituted mostly by the -CH2- group.
3.44: the downfield singlet of 3H represent m, the methoxy group with no neighbors.
1.50: n, the splitting pattern is exhibited for the same reason as m, but the chemical shift is more upfield due to weaker inductive effects.
Assignments (ppm) |
Chemical Shift |
Multiplicity |
J-Value |
Integration |
a |
7.41-7.43 |
m |
|
15H |
b |
5.10 |
d |
2.5 |
1H |
c |
4.92 |
d |
10.8 |
1H |
d |
4.83 |
d |
12.2 |
1H |
e |
4.76 |
d |
12.2 |
1H |
f |
4.63 |
d |
10.8 |
1H |
g |
4.53 |
s |
|
2H |
h |
4.11 |
dd |
4.1 |
1H |
i |
4.02 |
dd |
9.5, 9.5 |
1H |
j |
3.7 |
dd |
|
1H |
k |
3.76-3.71 |
m |
|
2H |
l |
“ “ |
“ “ |
|
“ “ |
m |
3.44 |
s |
|
3H |
n |
1.50 |
s |
|
3H |
 |