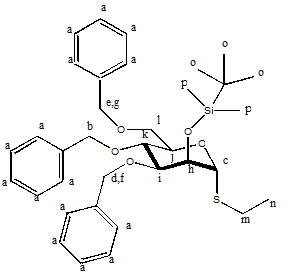
Explanation of Assignment of H NMR Peaks for Molecule 27
d7.2-7.4: a, the amount of hydrogen atoms (15) as well as the chemical shifts are clearly indicative of three mono-substituted aromatic rings.
5.10: b, these values are very high as Hb is flanked by an oxygen atom and a benzene ring. Also, they lie between two other identical, electronegative OBz groups, and are most effected by induction through the sigma bond structure based on their position relative to electronegative atoms.
4.72, 4.38: c is the highest value on the six member ring as it is adjacent to several electronegative atoms. It is also a doublet, and the coupling constant suggests that its lone neighbor, h, which has a lower chemical shift, must also be a ring bound hydrogen. Hydrogen h also exhibits only a doublet splitting pattern likely because it forms a dihedral angles of approximately 90o with Hi.
4.60, 4.50: These values represent d and f, which have identical coupling constants. The high values result from the contiguous oxygen atom and benzene ring. They are more downfield than the values corresponding to e and g because they are less removed from the six member ring.
4.58, 4.40: These values represent e and g, which have identical coupling constants. The high values result from the contiguous oxygen atom and benzene ring. See previous assignment for more information.
4.10: i, which has no coupling with Hh likely because of the Karplus Equation. Why it does not show coupling with Hk is unknown.
4.07: j, which is visible as a multiplet because it has 3 separate three bond neighbors, two of which are found on the adjacent -CH2-.
3.95: k, the similar coupling constants for the two doublets signify very similar neighbors. These correspond to Hi and Hj which are both also in axial positions.
3.60-3.80: l, the high value corresponds to -OCH2- hydrogen atoms. The complex splitting pattern is likely two separate dd, which show up very close to one another. 3H’s are designated by this range, and the identity of the third is rather ambiguous.
2.52: m, which is more upfield than the other hydrogens next to electronegative atoms because the inductive effects of the sulfur atom are less pronounced.
1.25: the triplet splitting pattern and 3H integration could only correspond to n.
0.85: o, which consists of nine identical H’s having no neighbors, and has and very low chemical shift due to its removal from the ring and its proximity to a silicon atom.
0.09, 0.00, -0.02: p, which have very low values because of the inverse inductive effects of silicon. The 12H’s likely also signal hydrogen atoms within the o designation.
Assignments |
Chemical Shift (ppm) |
Multiplicity |
J-Value (Hz) |
Integration |
a |
7.2-7.4 |
m |
|
15H |
b |
5.10 |
d |
1,2 |
H-2 |
c |
4.72 |
d |
10.8 |
1H |
d |
4.60 |
d |
11.7 |
1H |
e |
4.58 |
d |
12.3 |
1H |
f |
4.50 |
d |
11.7 |
1H |
g |
4.40 |
d |
12.3 |
1H |
h |
4.38 |
d |
10.8 |
1H |
i |
4.10 |
s |
|
1H |
j |
4.07 |
m |
|
1H |
k |
3.95 |
dd |
9.4, 9.4 |
1H |
l |
3.60-3.80 |
m |
|
3H |
m |
2.52 |
m |
|
2H |
n |
1.25 |
t |
7.5 |
3H |
o |
0.85 |
s |
|
9H |
p |
0.09, 0.00, -0.02 |
3s |
|
12H |
 |