THE FRESH PRINCE
OF
CHEMISTRY
| DMdO | ||
| A Brief Lesson | 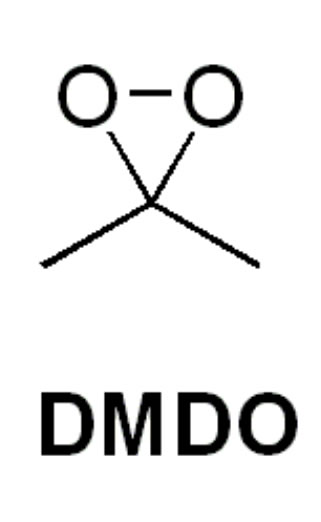 |
|
DMDO is a dioxirane derived from acetone. The DMDO acts as an electrophile in the reaction, with the alkene as the nucleophile. The geometry of the molecules creates a favorable interaction between the LUMO of DMDO and the HOMO of the alkene being oxidized. This reaction is favored additionally by hydrogen bonding that lowers the energy of DMDO’s LUMO. It is a favorable reagent to use because it is extremely mild and allows for oxidation to occur even when otherwise not possible. The primary by-product of this reaction is acetone, which is a relatively safe and desirable by-product.
|
||