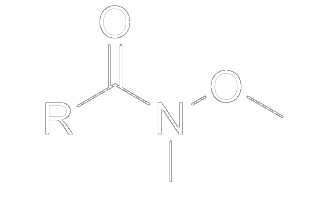
Weinreb amides are used in ketone and aldehyde synthesis [1]. A Weinreb amide takes the form of an amide, but with a methoxy group bonded to the nitrogen (N-methoxy-N-methylamides).

When a Weinreb amide is treated with an organometallic nucleophile, the reaction will go through a chelated intermediate. After adding acid, the chelated intermediate is hydrolyzed into a ketone.
The following mechanism demonstrates the reaction of a Weinreb amide with an organometallic reagent; the “M+” represents a metal anion.
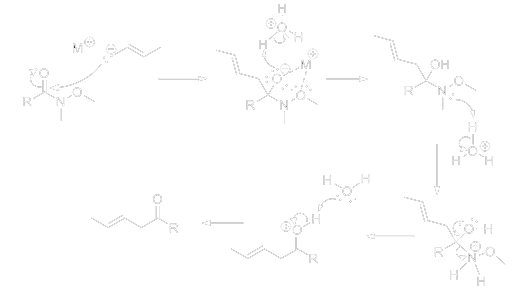
Weinreb amides have been proven to be useful and have a high efficiency largely due to the highly-stabilized tetrahedral chelate intermediate. The chelate intermediate effectively stops a second addition of the nucleophile [2]. This secondary addition would have produced a tertiary alcohol [3].
N-methoxy-N-methylamides have the benefit of being commercially available, relatively cheap, and also easy to handle and store [3].
[1] Hisler, K.; Tripoli, R.; Murphy, J. A. Tetrahedron Lett. 2006, 47, 6293-6295.
[2] Murphy, J. A.; Commeureuc, A. G. J.; Snaddon, T. N.; McGuire, T. M.; Khan, T. A.; Hisler, K.; Dewis, M. L.; Carling, R. Org. Lett. 2005, 7, 1427-1429.
[3] Nahm, S.; Steve M. Weinreb. Tetrahedron Lett. 1981, 22, 3815-3818.