
|
| In 210, we learned that a Palladium catalyst can be used to hydrogenate a C=C bond. This is a case of heterogeneous hydrogenation, meaning that the catalyst and the compound being hydrogenated are in two different phases of matter (i.e. one is solid and the other is liquid). Homogenous hydrogenation has the compound being hydrogenated and the catalyst in the same state of matter (in this case liquid). Homogenous catalysts are useful because they can often hydrogenate bonds that normal heterogeneous catalysts can not access due to steric hindrance. | |
Church, T.L.; Andersson, P. G. Coord. Chem. Rev. 2008. 252, 513-531. According to this article, the metal complex is oxidized through its reaction with H2 forming a separate bond with each hydrogen. The Iridium center subsequently coordinates with the double bond of the C=C bond you wish to hydrogenate. The first hydrogen is transferred through migratory insertion from the Ir-H bond to the C=C bond. Migratory Insertion basically means that either the hydrogen comes to the alkene or the alkene goes to the hydrogen. Scientists are not worried about which is the case, so they use migratory insertion to mean that one of those two scenarios occurred. After the migratory insertion, the catalyst is reduced when the next hydrogen transfers to the transformed C=C bond resulting in the hydrogenated product. The Iridium catalyst is first oxidized and later reduced, resulting in no net change in its oxidation number. |
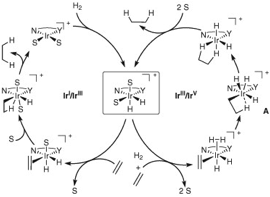 |
| Original Citation: Crabtree, R.H.; Davis, M.W. J. Org. Chem.1986, 51, 2655-2661. | ||
| These scientists also found the original article useful! | ||
| Song, Z.; Hsung, R.P.; Lu, T.; Lohse, A.G. J. Org. Chem. 2007, 72, 9722-9731. | ||
| Their attempts to hydrogenate C3 endocyclic olefin failed in the presence of a heterogeneous catalyst. However, by using Crabtree’s Catalyst, which was in the same state of matter as the olefin molecule, they were able to bypass any steric hinderance and easily hydrogenate the C3 endocyclic olefin. | ||
| Ramharter, J.; Mulzer, J. Org. Lett. 2009, 11,1151-1153. | ||
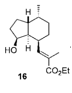
| They have used Crabtree’s catalyst to hydrogenate a double bond that was part of an enolate(15). In this case, they were able to facilitate syn addition to the double bond in the cyclohexene ring by using the hydroxyl group as an anchor for the Crabtree’s catalyst. This resulted in product 16. | 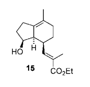 |
 |
Cui, X.; Burgess, K. Chem. Rev. 2005, 105, 3272−3296. |
||
| They have used Crabtree’s catalyst to hydrogenate tetrasubstituted alkenes. By doing this, they can create two chiral centers that are right next to each other. Crabtree’s catalyst is not enantiospecific and was able to give them the combination of products which they desired. | ||