The Sonogashira reaction is a coupling reaction with terminal alkynes with aryl (aromatic) or vinyl (ethenyl) halides. It is done under the presence of a palladium catalyst, a copper cocatalyst, and an amine base.
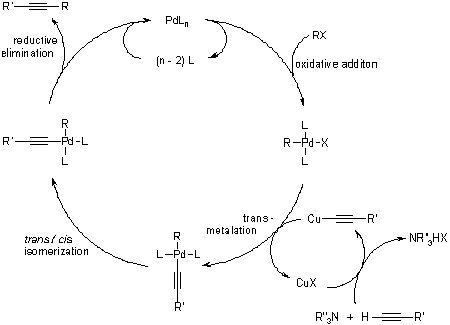
In the original paper by Sonogashira, the reaction requires two processes: the preparation of the Pd0 catalyst and the coupling of an aryl group from a halide species to an acetylene species. The copper cocatalyst allows the reaction to progress rapidly at room temperature. Today, the Pd0 catalyst no longer needs to be synthesized, since phosphine-palladium complexes like tetrakis(triphenylphosphine)palladium(0) can be used.
The Pd0 catalyst starts by combining with the alkyl halide through oxidative addition; specifically, an electrophilic oxidative addition. A metal with vacant coordination sites gets oxidized by the insertion of the metal into a covalent bond.
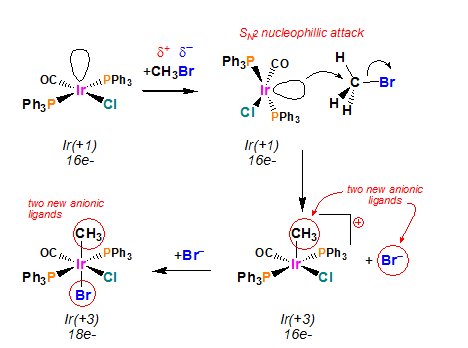
The H3C-Br bond is broken on the oxidative addition reaction generating two new anionic ligands: CH3- and Br-. If the starting metal complex is 16e- both ligands will usually end up coordinated to the metal to make an 18e- complex.
Both the oxidation state of the metal and the complexís electron count increases by two. It is noted that the oxidative addition of Rí-X to the Pd0 catalyst is made easier if the X group is iodide, because they are more electron-withdrawing.
The formation of the copper cocatalyst is the least understood of all the steps. The main limitation of this mechanism is that it canít account for the deprotonation of the terminal alkyne, because the base employed simply isnít basic enough. It is suggested that a pi-alkyne complex forms, followed by a deprotonation by the base, to form a copper acetylide.
The transmetallation of copper acetylide to the palladium is believed to be the rate-determining step. Transmellation describes the exchange of ligands between two metal centers. It is similar to the double displacement reaction.
The complex undergoes a trans/cisisomerization, as both ligands are trans and eventually convert to cis. This occurs relatively spontaneously. Since many isomers are roughly equal in bond energy, they can interconvert relatively freely.
This is leaves the alkyl and acetylene groups on the palladium center, which then go through reductive elimination. This is the opposite of oxidative addition, and it is favored when the bond formed is exceptionally strong.
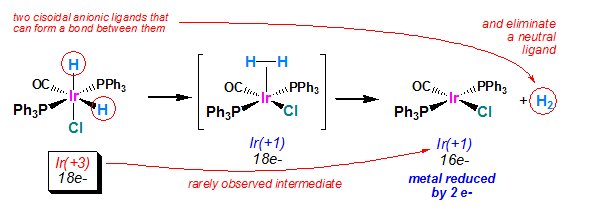
In order for reductive elimination to occur, the two groups need to be next to each other in the metalís coordination sphere. Hence, the trans-cisisomerization. This is the final step, yielding the coupled product and the Pd0 catalyst.
The solvent must be basic in order to neutralize the hydrogen halide produced as the byproduct, so alkylamine compounds such as triethylamine and diethylamine are typically used. DMF or ether can be used as solvent. Also, because the palladium(0) complexes are unstable in the air, classic Sonogashira coupling needs to be performed in the absence of oxygen.
Sonogashira coupling has been used in the syntheses of many compounds to minimize necessity for protecting groups and intermediates. The compound Phorboxazole A, which has shown high cytostatic and antifungal potential, was synthesized through Sonogashira coupling with no protecting groups and was modestly successful. Additionally, harveynone, a chemical isolated from the tea grey blight fungus Pestalotiopsistheae with anticancer properties,was first synthesized in a lab using Sonogashira coupling as its final step.
Although the innerworkings of the Sonogashira coupling reaction remain unclear, it is still very a useful reaction. New compounds and combinations of reactants could be used to identify paths that provide the lowest amounts of undesired side-coupling products. Research is currently concentrated on a copper-free Sonogashira reaction in order to minimize environmental pollution. The reaction holds a great potential for synthesizing future chemical compounds.
