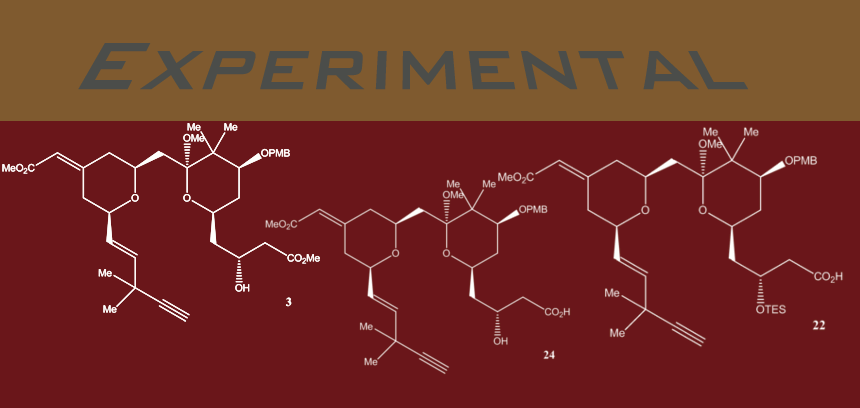
Step 1: Trimethyltin hydroxide (10 mg, 0.055 mmol) was added to a solution of compound 3 (16.5 mg, 0.025 mmol) in dry DCE (0.7 ml) under N2. The resulting mixture was stirred at 80 degrees C for 12 hours. Compound 24 was purified directly via silica gel flash column chromatography to give a colorless oil (13.5 mg, 84% yield).
Step 2: To a solution of hydroxyacid 24 (74 mg, 0.12 mmol) in DCM (2.5 ml) freshly distilled 2,6-lutidine (62 mg, 0.58 mmol) was added at -10 degrees C, followed by the drop wise addition of freshly distilled TESOTf (67 mg, 0.25 mmol). The resulting solution was stirred at the same temperature for 20 min, before poured into pH 7.0 buffer. The mixture was extracted with ethyl acetate five times and the combined organic fractions were dried over Na2SO4. The TES ether-acid 22 was purified via quick silica gel flash column chromatography to give a colorless foam.
Glossary Trimethyltin hydroxide 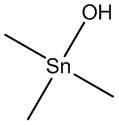 Used for the hydrolysis of esters.
Used for the hydrolysis of esters.
DCE (Dichloroethane) 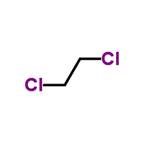 Chlorinated hydrocarbon used as a solvent.
Chlorinated hydrocarbon used as a solvent.
"Under N2"
Running a reaction under nitrogen means that the system constantly has nitrogen flowing through it. The solution will react with air, but not nitrogen so running nitrogen through the system means that there will be no unwanted reactions.
silica gel flash column chromatography 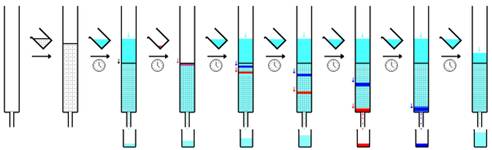 Silica gel flash column chromatography is a method used to purify individual chemical compounds from mixtures of compounds.
Silica gel flash column chromatography is a method used to purify individual chemical compounds from mixtures of compounds.
2,6-lutidine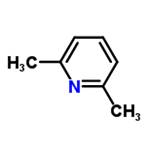 2,6-Lutidine is a natural heterocyclic aromatic organic compound. It is weakly nucleophilic, due to the steric effects of the two methyl groups on the ring nitrogen. It is moderately basic, with a pKa of 6.7. In organic synthesis, 2,6-lutidine is thus widely used as a sterically hindered mild base.
2,6-Lutidine is a natural heterocyclic aromatic organic compound. It is weakly nucleophilic, due to the steric effects of the two methyl groups on the ring nitrogen. It is moderately basic, with a pKa of 6.7. In organic synthesis, 2,6-lutidine is thus widely used as a sterically hindered mild base.
TESOTf 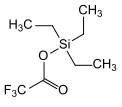 Used as a catalyst in reaction.
Used as a catalyst in reaction.