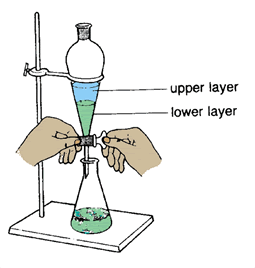
Experimental Glossary: Extraction
Extraction is a method of separating organic molecules out from those that are not organic. This method employs the usage of a separatory funnel. Typically, organic solvents (such as dichloromethane) and aqueous solvents (such as HCl) are used. In a separatory funnel, the organic solvent and its dissolved substances will form a distinguishable layer from the aqueous layer and its dissolved substances. Whether or not the organic layer is above or below the aqueous layer in the separatory funnel depends on its density relative to the density of the aqueous layer. If dichloromethane and HCl are used, the organic layer will be below the aqueous layer because dichloromethane is more dense, therefore heavier, than HCl. This apparatus can be seen in the diagram below.
Once the two layers are discernable, they can be released from the separatory funnel and collected into their respective containers. To ensure the purity of the extracted organic layer, extractions can be performed multiple times.