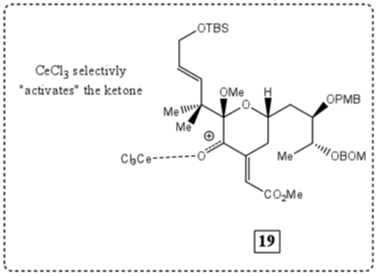
Explanation on the observed regioselective and stereoselective outcome of the Luche reduction of 19
While the Luche reduction has been tested for over 30 years, a clear explanation for the stereoselective outcomes of compounds which undergo this sort of reduction is still vague and unknown. The novel characteristic of the Luche reduction is its ability to not affect specific groups such as carboxylic acids, esters, amides, halides, and cyano and nitro groups. When looking at compound 19 from the previous reaction, it may be noted that the only “available” site for reduction via the Luche method is the ketone found on the 6-membered, oxygen containing ring.
Why is only this ketone (and only ketones in general) affected? While many explanations have been brought forth, the simple concept of the Hard-Soft Acid and Bases (HSAB) theory has been the most supported explanation. While hard acids or bases are compact, with the electrons held fairly tightly by the nucleus, and not very polarizable; soft acids and bases are larger, with a more diffuse distribution of electrons. Knowing this, the HSAB simply states that hard acids react preferentially with hard bases, and soft acids react preferentially with soft bases.
In the case of a Luche reduction reaction, the lanthanide (Ce3+ in this case) acts to increase the electrophilicity of the specific ketone-carbonyl group. This CeCl3-O complex allows for the hardness of the borohydride (NaBH4) to increase by replacing hydride groups with alkoxide groups. Because of this increase in “hardness” the NaBH4 can now be considered a “hard” acid. Along with this, ketones have been categorized as a “hard” Lewis base. Following the HSAB theory, when the unstable CeCl3 activates the ketone, the C=O becomes delocalized (shown below). This activation causes the carbon center of the ketone to become partially positive and highly basic. The “hard (acid)” NaBH4 complex is then allowed to protonate the carbon center, causing the double bond to be moved onto the partially positive oxygen atom. With the help of methanol, the O-CeCl3 “ionic-like” bonding is destroyed by further protonation to give the final alcohol intermediate.
But why are only ketones affected while other C=C or C=O containing groups are not? Going back to the HSAB theory it is noted that “hard acids react preferentially with hard bases, and soft acids react preferentially with soft bases.” Since the newly created NaBH4 “complex” is considered a hard acid, it will preferably only associate and react with a subsequent “hard” base. In this regard, the ketone is the only hard base found in molecule 19. Ketones are small, and having weak nucleophilicity, are allowed to react with the CeCl3 compound. In regards to the Luche reduction, the HSAB theory can also explain the inability of the Luche reaction to reduce esters. When looking at the structure of an ester it is noted that additional resonance contributions delocalizes the C=O electrons to a larger area. Compared to the C=O in a ketone, this diffuse distribution of electrons categorizes the ester as a “softer” base in comparison to the “hard” basicity of the ketone. All in all, compared to other groups such as carboxylic acids, esters, amides, halides, and cyano and nitro groups, ketones are considered “harder” bases which in turn allow them to participate with “hard” acids.
In the case of the stereoselectivity of the Luche Reduction, the main thing to remember is that the formation of the C-H sigma bond is axially favored. In this case, when the CeCl3 attaches to the oxygen atom, the carbon center becomes highly electrophilic. Because of this electrophilic nature, a nucleophilic hydride anion is allowed to approach the carbon center either equatorially or axially. The hydride anion selectively prefers to approach and attach axially. This is manly because the stabilization effects that are created from the adjacent pi-system. When the hydride anion creates a bond axially, the p-orbitals of the adjacent system are parallel to that bond and hydrogen. This parallel nature allows the p-orbitals to delocalize/stabalize the charge that the anion brings with it.