

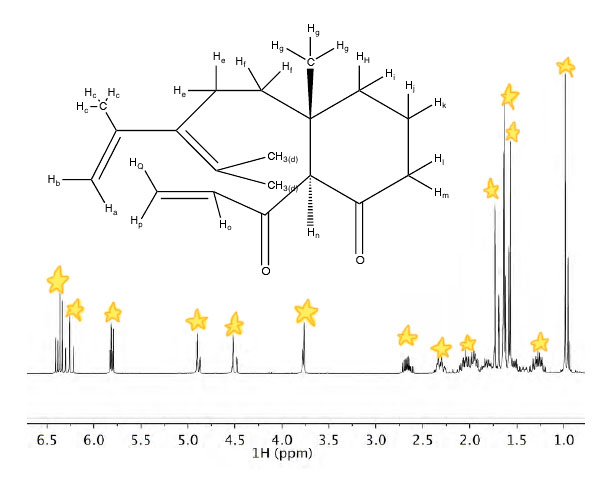


Hydrogens |
Explanation |
a |
The shift at 4.52ppm represents hydrogen a. This hydrogen is a doublet of quartets. It is more downfield and less shielded than the majority of the other hydrogens due to the double bond. It has coupling constants of 2.7Hz and 0.9Hz. |
b |
The shift at 4.90ppm represents hydrogen b. This hydrogen is a doublet of quartets. It is more downfield and less shielded than hydrogen a since it is cis to the –CH3 group. It has coupling constants of 3.0Hz and 1.5Hz. |
c |
The shift at 1.73ppm represents the hydrogens labeled c. These three hydrogens are a doublet of doublets. These hydrogens have 2 neighbors. The neighbors of these H’s are Hb and Ha. They have coupling constants of 1.5Hz and 0.9Hz. These hydrogens are shielded, but not as shielded as hydrogen d because the hydrogens labeled d are terminal hydrogens. |
d |
The shift at 1.57ppm represents hydrogen d. These six hydrogens are a singlet. These hydrogens are shielded because they are terminal hydrogens that are not near any electronegative atoms. |
e |
The shift at 2.68ppm represents hydrogen e. This hydrogen is a doublet of doublet of doublets. This hydrogen has coupling constants consist of of 14.2Hz, 10.6Hz, and 6.8Hz. They are no near any withdrawing electrons, so they are shielded; although, they are not as shielded as hydrogen m. |
f |
The shift at 1.35-1.15ppm represents the hydrogens labeled f. These two hyrogens are a multiplet. These hydrogens have two neighbors. These hydrogens are shielded because there not any electrons withdrawing from hydrogens. |
g |
The shift at 0.99ppm represents the hydrogens labeled g. These hydrogens are a singlet. They are the furthest upfield because they are the most shielded of any of the H’s on the molecule. They have no neighbors. |
h, j, l |
The shift at 2.20-1.80ppm represents hydrogen(s) h,j, and/or l. These five hydrogens are a multiplet due to their location in a ring. These hydrogens are shielded and do not have withdrawing electrons. |
k, i |
The shift at 1.55-1.45ppm represents hydrogen k or i. This hydrogen is a multiplet. These hydrogens are part of a muliplet because they are in a ring. These hydrogens are relatively shielded because they are not by any electronegative atoms that would tend to withdraw electrons. |
m |
The shift at 2.35-2.25ppm represents hydrogen m. This hydrogen is a multiplet due to its location in a ring. The hydrogen is shielded and does not have any withdrawing electrons. |
n |
The shift at 3.76ppm represents hydrogen n. This hydrogen is a triplet. Hydrogen n is near two oxygens, so that is why the peak is more downfield than all of the peaks that are more upfield than hydrogen n. It has a coupling constant of 1.2Hz. |
o |
The shift at 6.35ppm represents hydrogen o. This hydrogen is a doublet. It is located the farthest downfield due to the proximity to the oxygen. It is also near a double bond which makes it more downfield. It has a coupling constant of 10.2Hz. |
p |
The shift at 5.80ppm represents hydrogen p. This hydrogen is a doublet. This hydrogen is downfield and deshielded due to the double bond. It is more upfield than Q because it is cis to hydrogen o. It has coupling constants of 10.3Hz and 1.1Hz. |
Q |
The shift at 6.24ppm represents hydrogen Q. This hydrogen is a doublet. This hydrogen is downfield and deshielded due to the double bond. It is more downfield than p because it is trans to hydrogen o. It has coupling constants of 17.4Hz and 1.0Hz. |