Diels-Alder Reaction
The Diels-Alder reaction is a cycloaddition reaction in which a dienophile attacks a conjugated diene. The diene can be open-chain or cyclic and can
have many different kinds of substituents, but it must be cis to react.
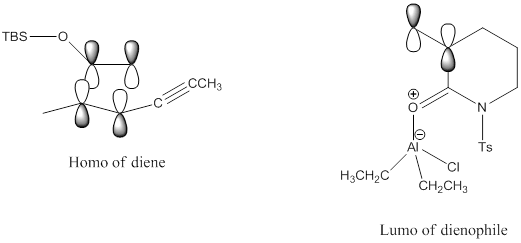
Therefore, dienes that favor the cis conformation are more reactive in Diels-Alder than those that favor the trans conformation. The overall reaction is concerted, which means that all bonds break and form in a single step. Diels-alder reactions are thermal, which means that the HOMO of the diene and the LUMO of the dienophile are used. When a Lewis acid is added, it activates the dienophile by making the dienophile more electron deficient and lowering its LUMO. When this happens, since LUMO is a higher energy level than HOMO, the two levels get closer together and it is easier
for the reaction to occur. In addition, lowering the reaction temperature
to -78 Celsius Degree improves the yield by preventing side polymerization reactions. According to FMO theory, N-tosyl lactam will be the LUMO in the reaction (from TBS enol ether (E) to N-Tosyl spirolactam), the energy of LUMO of C=O double bond is higher than the energy of LUMO of C=C double bond because O is more electronegative than C. Therefore, the C=C double bond will be the dienophile in the reaction to react with the diene because C=C double bond energy level is closer to the energy level of the HOMO of the diene.