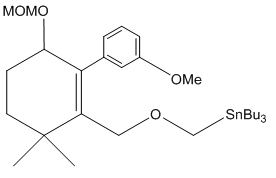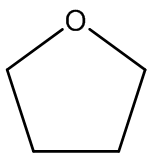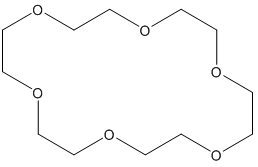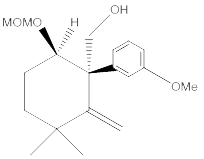
Compound 8: An oven-dried round-bottomed flask was charged with KH (360 mg, 9 mmol) and 20 mL of THF under argon at 0°C. A solution of compound 7 (920 mg, 3 mmol) in 5 mL of THF was injected slowly into the flask. Thirty minutes later, 18-crown-6 (780 mg, 3 mmol) was added. The reaction mixture was stirred for additional 5 min and then α-iodomethyl tributylstanne (3.9 g, 9 mmol) was injected. The reaction mixture was stirred at this temperature for 6 hours. After quenching with water carefully, the product was extracted with ether (3 x 20 mL) and dried over MgSO4. The drying agent was removed by filtration and the solvent was concentrated using rotovap. The residue was purified by flash chromatography, and the desired product was dissolved in THF under argon. The mixture was cooled to -78°C and BuLi in hexane (3.6 mL, 9 mmol) was injected dropwise. The temperature was slowly warmed up to -20°C in 6 hours and the reaction was quenched with saturated NH4Cl solution, extracted with ether (3 x 30 mL), and dried over MgSO4. After filtration, the extract was concentrated and the residue was purified by chromatography to afford compound 8 (0.78g, 80% for 2 steps). 1H NMR (CDCl3, 400 MHz): δ 7.25-7.14 (m, 3H), 6.75-6.71 (m, 1H), 5.37 (s, 1H), 5.34 (s, 1H), 4.78 (d, J = 10.4 Hz, 2H), 3.96 (dd, J = 16.0, 6.4 Hz, 1H), 3.84-3.79 (m, 2H), 3.78 (s, 3H), 3.45 (s, 3H), 3.26 (dd, J = 9.6, 9.6 Hz, 1H), 2.27 (dddd, J = 16.4, 16.4, 16.4, 6.8 Hz, 1H), 1.96 (dddd, J = 17.6, 6.0, 6.0, 6.0 Hz, 1H), 1.46-1.31 (m, 2H), 1.10 (s, 3H), 0.52 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 159.0, 154.6, 143.0, 128.5, 121.4, 115.4, 110.8, 110.7, 96.8, 80.5, 66.5, 56.3, 55.9, 55.0, 38.5, 36.9, 31.3, 30.1, 24.9; IR (neat): cm-1 3485, 2953, 1606, 1579, 1485, 1464, 1053, 1027.
Works Cited
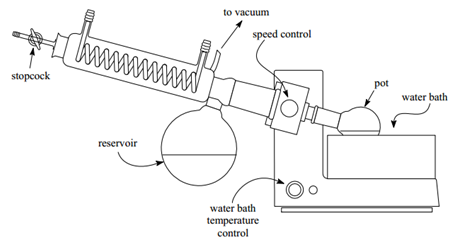
- Flash Chromatography. http://yvesrubin.files.wordpress.com/2011/03/flash_chromatography.pdf (accessed Apr 10, 2013).
- MPN Organic. Rotary Evaporator. http://mirandamusic.com/mpnorganic/rotovap.html (accessed Apr 10, 2013).