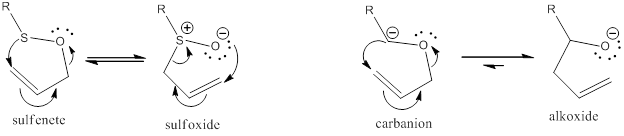
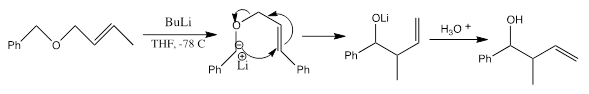
A [2,3]-Wittig rearrangement reaction is a [2,3]-sigmatropic rearrangement reaction with high stereoselectivity. The reaction synthesizes homo-allylic alcohols through the base-induced rearrangement of allyl ethers at low temperatures. The rearrangement presents a thermal isomerization proceeding through a six-electron, five-membered ring transition state. The reaction is very similar to the [1,2]-sigmatropic reaction. For this reason, the reaction is conducted at low temperatures to avoid contamination by the [1,2]-rearrangement product. A [2,3]-rearrangement feature regioselective carbon-carbon bond formation along with allylic transposition of the oxygen and chirality transfer. The reaction rate depends on the energy gap between the LUMO (allyl) and the HOMO (anion). Additionally, the less stable carbanion, the faster the rearrangement.

>