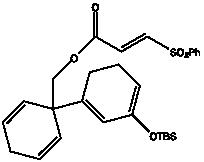
Compound 26
Compound 25(513 mg, 1 mmol) was dissolved in 50 ml of anhydrous toluene in a high-pressure tube. The reaction mixture was degassed with argon and then stirred for 170 ºC for 1 hour. After cooling to room temperature and [sic] the mixture was transferred to a round bottom flask and the solvent was removed with rotavap. The residue was dissolved in THF and cooled to 0 ºC. Then TBAF in THF (1.5 ml, 1.5 mmol) was added. 2 hours later the reaction was quenched with saturated ammonium chloride solution, extracted with ethyl acetate (3 x 10 ml) and dried with MgSO4. The drying agent was removed by filtration and the solvent was removed under reduced pressure. The residue was purified using flash chromatography. (156 mg, 62%) 13C NMR (CDCl3, 100 MHz): ! 208.5, 163.5, 143.1, 135.7, 129.3, 128.6, 124.4, 123.3, 71.7, 50.1, 46.5, 41.3, 38.9, 26.9, 25.9, 23.1; IR (neat): cm-1 2946, 1720, 1616, 1242, 1075; HRMS (FAB, m/z) calcd for C16H17O3 [M + H]+ 257.1178, found 257.1178

Glossary
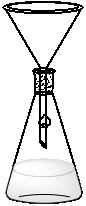
A high yield and tensile strength tube with resistance to high pressures. It is used in a variety of high-pressure applications involving high-pressure liquid or gas systems.
Degassing is a technique used to remove oxygen and other reactive gases from a reaction mixture, consisting of bubbling an inert gas, such as nitrogen or argon, through the solvent for a period of 30 minutes to 1 hour. This technique is often essential in reactions that require excessive or prolonged heating, or contain electron-rich aromatic compounds.

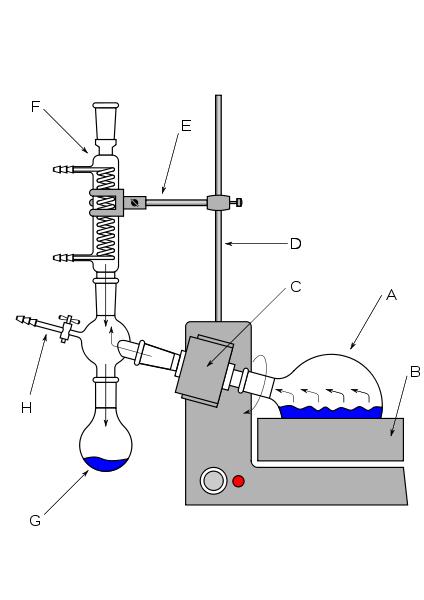
A rotary evaporator, commonly known as a rotavap, is a device used to remove solvent from systems by reducing pressure, increasing temperature, and increasing surface area. This allows for a quicker evaporation time, at a lower temperature than would normally be possible, avoiding the possible decomposition of unstable products.
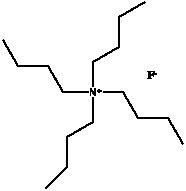
Tetra-n-butylammonium fluoride. Used in the deprotection of alcohols via the hydrolysis of alkyl silyl ethers.
A common polar aprotic solvent used in organic systems that react with protic solvents.
Filtration used to remove solid impurities from an organic liquid. The mixture is poured into a pre-wetted, liquid permeable, filter, placed in a stemmed funnel, which then drains into the desired receptacle, leaving behind the solid waste.
Flash chromatography, also known as medium pressure chromatography. It differs from conventional column chromatography in the usage of smaller silica gel particles (250-400 mesh). In addition, pressurized gas (10-15 psi) is used to drive the solvent through the column. This results in a much faster, high-resolution chromatography.
Sources:
Bode, Jeffery. "How to Degas Solvents." University of California Department of Chemistry. University of California, Santa Barbara, n.d. Web. 28 Mar. 2013. <http://chem.rochester.edu/~nvd/howtodegas.html>.
"Filtration." Organic Chemistry at CU Boulder. University of Colorado at Boulder, n.d. Web. 28 Mar. 2013. <http://orgchem.colorado.edu/Technique/Procedures/Filtration/Filtration.html>.
"Flash Chromatography." Yves Rubin Research Group. N.p., n.d. Web. 28 Mar. 2013. <http://yvesrubin.files.wordpress.com/2011/03/flash_chromatography.pdf>.
"High-pressure tubes." Sandvik. Sandvik, n.d. Web. 28 Mar. 2013. <http://www.smt.sandvik.com/en/products/tube-pipe-fittings-and-flanges/tubular-products/high-pressure-tubes/>.
"Rotary Evaporator." The Free Dictionary. Farlex, n.d. Web. 28 Mar. 2013. <http://www.thefreedictionary.com/rotary+evaporator>.
"Rotary Evaporator." Wikipedia Commons. Wikipedia, 30 Oct. 2009. Web. 28 Mar. 2013. <http://commons.wikimedia.org/wiki/File:Rotary_Evaporator.svg>.
"Tetrabutylammonium fluoride solution." Sigma-Aldrich. Sigma-Aldrich, n.d. Web. 28 Mar. 2013. <http://www.sigmaaldrich.com/catalog/product/aldrich/216143?lang=en®ion=US>.
"Tetrahydrofuran." Sigma-Aldrich. Sigma-Aldrich, n.d. Web. 28 Mar. 2013. <http://www.sigmaaldrich.com/catalog/product/sial/34865?lang=en®ion=US>.