m-CPBA(42 mg, 0.18 mmol) was added to a solution of 28 (33 mg, 0.12 mmol) in 5 mL anhydrous DCM at 0°C.
The reaction was allowed to proceed for 12 hours and then the ice-bath was removed. The reaction mixture was
then stirred at room temperature for 6 hours. It was monitored under TLCuntil the starting material
disappeared. Then the reaction was quenched with a Na2S2O3 solution, extracted with ethyl acetate and then dried
over MgSO4. The drying agent was filtered and the solvent was removed under reduced pressure. The residue was
purified using flash chromatography to afford 29 (25 mg, 72% yield)
1H NMR (CDCl3, 400 MHz): δ 5.72-5.67 (m, 1H), 5.22 (d, J = 10.8 Hz 1H), 4.72 (d, J = 11.6 Hz, 1H),
4.30 (d, J = 11.6 Hz, 1H), 4.15-4.13 (m, 1H), 3.40-3.39 (m, 1H), 2.82-2.72 (m, 2H), 2.62 (dd J = 18.4, 5.6 Hz, 1H),
2.51-2.41 (m, 3H), 2.10 (d, J = 18 Hz, 1H), 2.00-1.65 (m, 5H)
meta-chlorobenzoic acid m-CPBA is a well known peroxy acid used often in epoxidation reactions.
DCM
Dichloromethane A common halogenated solvent.
TLC: Thin Layer Chromatography
TLC is a chromatography technique used to determine the number chemicals and their relative
polarity in a solution. TLC utilizes the difference in polarity of compounds by allowing a solvent
system to travel up the plate and carry the compounds with it. Those with higher polarity will “stick”
to the plate more readily and appear lower in the plate.
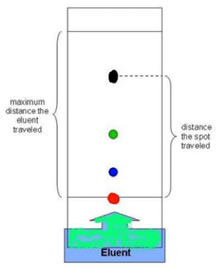
An example of a TLC plate that has been run with a solution
of four compounds (Red, Blue, Green, and Black)
Flash chromatography is a method used to purify compounds from mixtures and impurities.
The process is a type of column chromatography. However a pump or compressed gas is used to
expedite the process. Silica gel is used to separate the mixture and the solvent is collected in a flask
(as shown below). A pumped is attached to the top of the apparatus and is used to push the solvent
through the silica gel to accelerate what would usually take much longer if solely relying on gravity.