Leading Question
Butch Coolidge wants to know how SeO2 is used in organic syntheses.

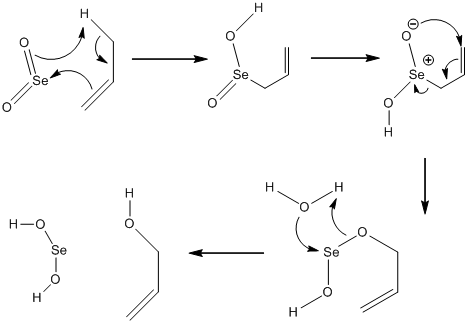
Selenium dioxide (SO2) is a colorless compound that is often used as an oxidizing agent in organic synthesis reactions. It is often used for allylic oxidation, when an OH replaces a carbon such as the above mechanism.
For our mechanism, it is slightly different. Two hydrogens are removed from a six-membered carbon ring and a double bond forms in their place. One of the oxygens on the selenium dioxide breaks its bond with selenium and deprotonates a carbon on the ring. Then the bond between the ring and the remaining hydrogen break, as that hydrogen and the selenium form a bond.