Papers Relating to Mechanism
#1
Trost, B. M. Comprehensive Organic Synthesis; Pergamon Press: Oxford, 1991; Collect. Vol. No. 8, pp 524-545.
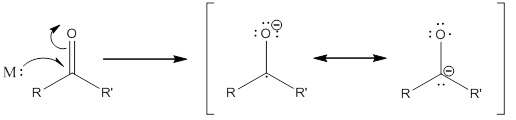
This book explains several important features of our mechanism, namely the initiation of the reaction. In the book, it is explained that aluminum amalgam and other dissolving metals involved in reduction will donate an electron to a vacant orbital of the substrate. This will result in the formation of the structures below.

Following this, another electron is added to the substrate which creates a dianion. The electrons on the carbon atom will facilitate the formation of an enolate anion by forming a double bond in the ring, and will cause the carbon-oxygen bond nearby to break in the process. In this way, the epoxide undergoes a ring-opening. The negatively charged oxygen is then protonated and the lone pair of the enolate oxygen atom forms a double bond with the nearby carbon, thereby restoring the carbonyl and moving the electrons of the double bond to pick up a proton. Because the carbonyl is restored, the process happens once more to open the epoxide on the opposite side.
#2
Larock, R.C. Comprehensive Organic Transformations, 1st ed.; Wiley: New York, 1989; pp 505-508.
#3
Solladie, G.; Hutt, J. J. Org. Chem. 1987, 16, 3560-3566.
References Citing Reference #1
1. Petroski, R. J. Synthetic Commun. 2011, 41, 63-66.
-The author chose to cite this work because the procedure uses aluminum amalgam to reduce a carbonyl, resulting in an epoxide ring-opening.
2. Nair, V.; Poonoth, M.; Vellalath, S.; Suresh, E.; Thirumalai, R. J. Org. Chem., 2006, 71, 8964–8965.
-The author chose to cite this work as the synthesis involves an amalgam and the opening of an epoxide near a carbonyl group.
3. Wang, Y. C.; Yan, T. H. Chem. Commun. 2000, 7, 545.
-The author chose to cite this work as an aluminum amalgam was used to exact a very similar transformation involving the opening of an epoxide near a carbonyl.
Other References
Kwon, M. S.; Park, I. S.; Jang, J. S.; Lee, J. S.; Park, J. Org. Lett., 2007, 9, 3417-3419.
Murphy, D.K.; Alumbaugh, R. L.; Rickborn, B. J. Am. Chem. Soc., 1969, 91, 2649–2653.
Sweeting, Linda M. “Reducing Agents.” Oct. 2001. 3 Apr. 2013 http://pages.towson.edu/ladon/orgrxs/reagent/reducers.htm.
1,5-HEXADIENE DIEPOXIDE | 1888-89-7. (n.d.). ChemicalBook---Chemical Search Engine. Retrieved April 3, 2013, from http://www.chemicalbook.com/ChemicalProductProperty_EN_CB7422292.htm
Abstract for TR-362 - 4-Vinyl-1-cyclohexene Diepoxide (CASRN 106-87-6) - National Toxicology Program. (n.d.). Home - National Toxicology Program. Retrieved April 3, 2013, from http://ntp.niehs.nih.gov/?objectid=0708B9DB-D780-96F2- D7EF48799058ECDA
Column Chromatography. (n.d.). Organic Chemistry at CU Boulder. Retrieved April 3, 2013, from http://orgchem.colorado.edu/Technique/Procedures/Columnchrom/Column chrom.html
Filtration Diagram - Pictures, Photos & Images of Chemistry - Science for Kids. (n.d.). Science for Kids - Fun Experiments, Cool Facts, Online Games, Activities, Projects, Ideas, Technology. Retrieved April 3, 2013, from http://www.sciencekids.co.nz/pictures/chemistry/filtrationdiagram.html
Column chromatography. (n.d.). chemguide: helping you to understand Chemistry - Main Menu. Retrieved April 3, 2013, from http://www.chemguide.co.uk/analysis/chromatography/column.html
