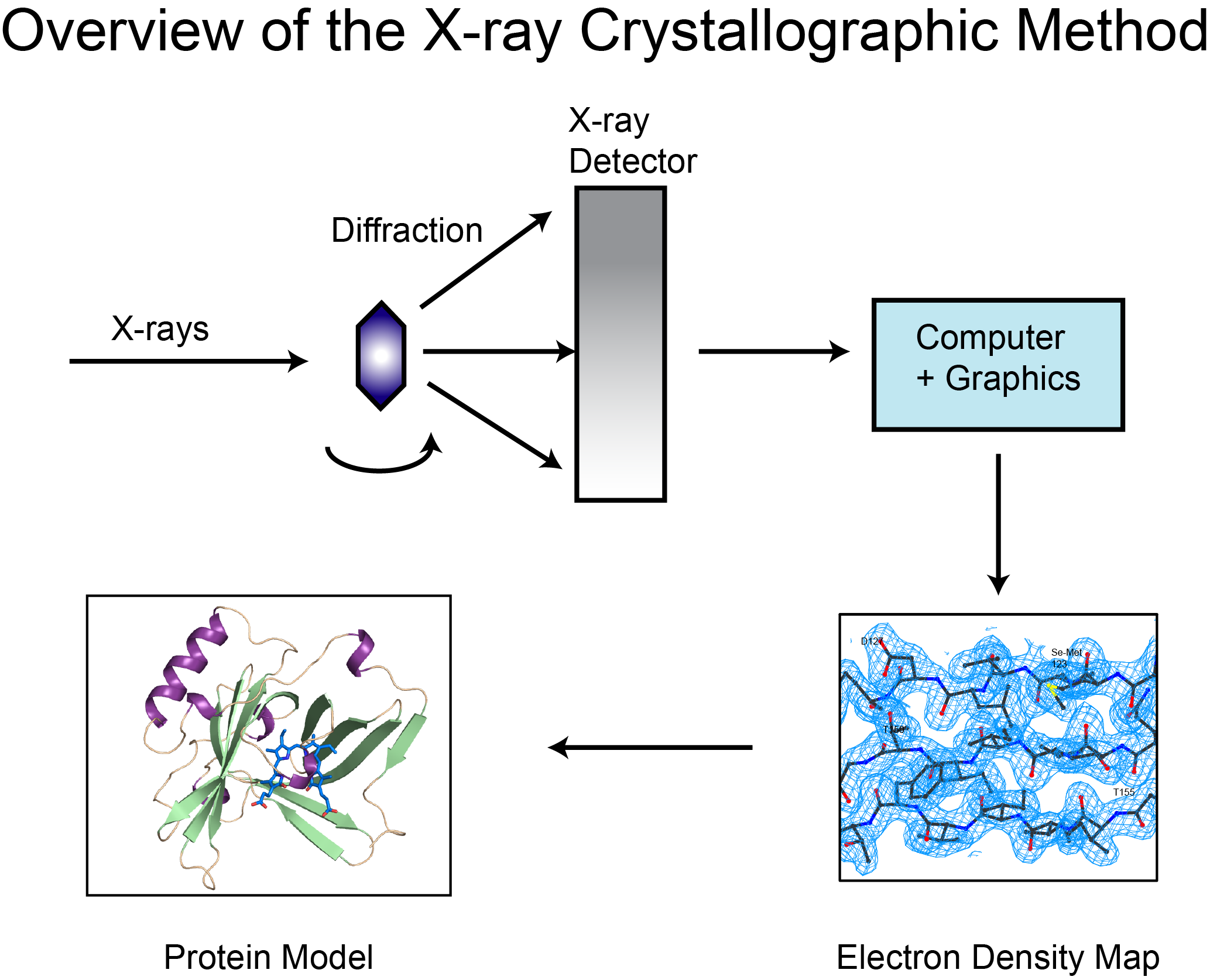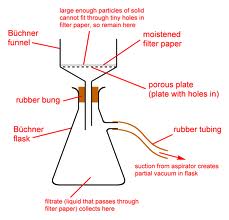
Nitrile Lactone (+)-41: A solution of freshly distilled diisopropylamine (18 mL, 128 mmol) and tetrahydrofuran (200 mL) was cooled to 0 °C in an ice bath, and a solution of n-butyllithium in hexanes (2.16 M, 50 mL, 108 mmol) was added dropwise at 0 °C. The reaction mixture was stirred at 0 °C for 30 minutes. A solution of freshly distilled lactone (–)-32 (7.36 g, 48.36 mmol) in tetrahydrofuran (25 mL) was added at 0 °C, and the reaction mixture was allowed to return to room temperature. After 2 hours the reaction mixture was cooled to 0 °C and a solution of cyanobenzotriazole (10.2 g, 70.77 mmol) in tetrahydrofuran (25 mL) was added dropwise at 0 °C. The reaction mixture was allowed to return to room temperature and was stirred overnight. The reaction mixture was diluted with hydrochloric acid (400 mL, 1N aqueous) and stirred at room temperature for 10 minutes. The organic layer was separated, and the aqueous layer was washed with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate. The solvent was removed in vacuo and the resulting residue was dissolved in diethyl ether (200 mL), washed with hydrochloric acid (1N aqueous), and stirred with sodium bicarbonate (200 mL, saturated aqueous) for 40 minutes. The aqueous layer was washed twice with diethyl ether, acidified to pH < 1 with hydrochloric acid (concentrated aqueous), set aside for 10 minutes, and extracted with dichloromethane (5x, 250 mL total). The combined organic extracts were dried over anhydrous sodium sulfate. The solvent was removed to give nitrile lactone (+)-41 (5.1 g, 28.8 mmol, 60 %, > 90% purity). A spectroscopically pure sample could be obtained by trituration from dichloromethane using hexanes. The crystals were filtered off and rinsed with hexanes then air-dried. X-ray quality crystals were prepared by vapor diffusion (dichloromethane / hexanes): melting point 104-106 °C; ), IR (film) 3033 (w), 2252 (w), 1730 (s), 1404 (m), 1240 (s), 1221 (s) cm-1; 1H-NMR (500 MHz, CDCl3) 5.77-5.72 (m, 1H), 5.72-5.67 (m, 1H), 4.48 (dd, J = 11.5, 3.5 Hz, 1H), 4.32 (dd, J = 11.5, 3.1 Hz, 1H), 3.62, (d, J = 11.1 Hz, 1H), 2.70-2.63 (m, 1H), 2.55-2.47 (m, 1H), 2.38-2.31 (m, 1H), 2.30-2.20 (m, 2H), 2.11-2.02 (m, 1H); 13C-NMR (125 MHz, CDCl3) ! 162.7, 124.9, 123.2, 115.9, 73.8, 35.9, 34.1, 29.0, 27.8, 23.1.
Terms explained:
In vacuo: literally in a vacuum, refers to standard vacuum filtration used to dry and remove solvent from solids. In our reaction, used twice to separate solvent from solid.

Trituration: In organic chemistry, a method to purify compounds by removing bound impurities. A solvent is chosen in which the desired product is very insoluble and the impurities are very soluble. The material is washed with the solvent of choice and the product is purified of impurities, which dissolve in the solvent. The remaining solid is the purified desired compound.


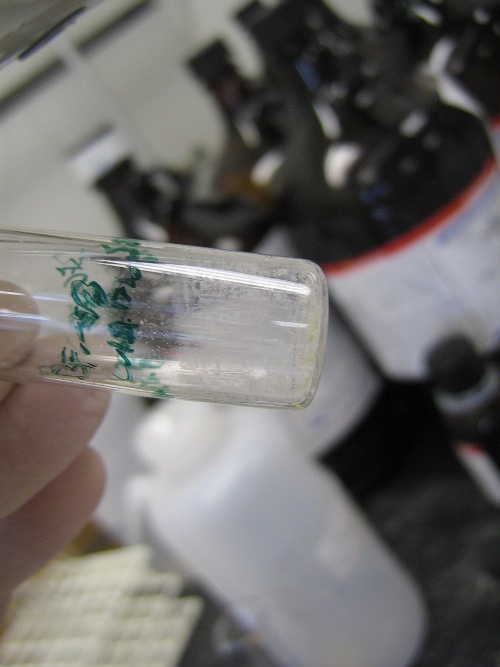
X-Ray Quality Crystals: crystals that are suitable for X-Ray crystallography. This is a way of determining the atomic and/or molecular structure of a crystal by measuring angles of diffracted X-Ray light.