Leading Question
Other reagents used to introduce CN group & why BtCN was used:
BtCN (cyanobenzotriazole) was most likely used as a reagent in our reaction because it’s a good source of CN+ that can be used to make α-cyano-sulfones, -ketones, -alkanecarboxylate esters, -cyanides, -alkylheterocycles, -diarylmethanes and heterylcarbonitries. For each of these, BtCN has a yield between 55 and 78%, which is a good to high yield. Other reactions that introduce a cyano group use organometallic reagents with tosyl cyanide, 2-chlorobenzyl thiocyanata, thiocyanogen, 2-cyanopridazin-3(2H)-ones, cyanogen alides, or cyanoimidazole. Gallium, for example, is a poor metal found to be able to catalyze direct cyanation reactions. However, these reagents can problems with stability, lack of reactivity, poor solubility, corrosiveness, availability, toxicity, cost or can have complicated preparative procedures.
Katritzky, A. R.; Akue-Gedu, R.; Vakulenko, A. V. Arkivoc 2007, iii, 5−12.
Okamoto, K.; Watanabe, M.; Murai, M.; Hatano, R.; Ohe, K. Chem. Commun. 2012, 48, 3127-3129.
Example:
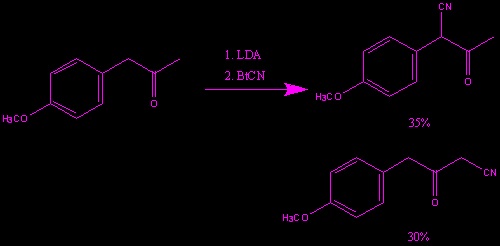
This is an example of BtCN in action. In summary, the use of cyanobenzotriazole in a broad variety of heterocyclic and functionalized a-nitriles has been established to be an efficient methodology. It is advantageous cyanation reagent because it is stable, non-volatile, crystalline solid that limits the risk of possible toxic exposure.
Katritzky, A. R.; Akue-Gedu, R.; Vakulenko, A. V. Arkivoc 2007, iii, 5– 12


