Speculate as to why the benzyl instead of benzoyl protection of the phenylhydrazone resulted in the more efficient formation of the indole system.
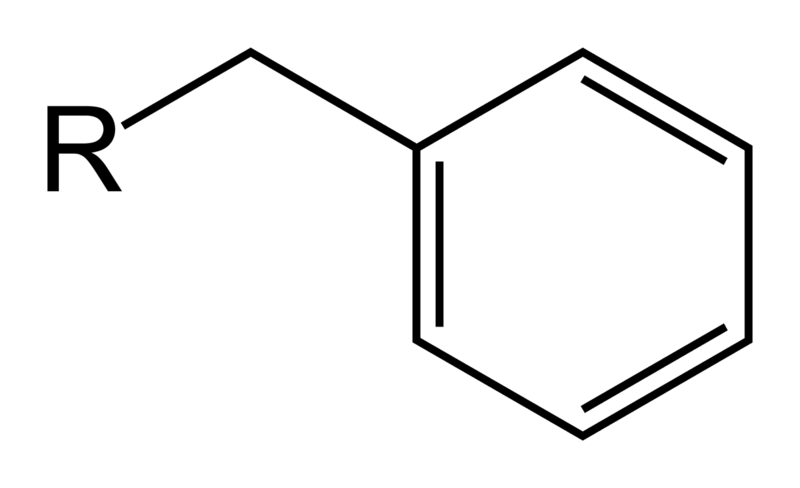
Benzyl
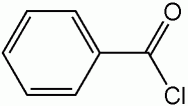
Benzoyl
Leading Question
Speculate as to why the benzyl instead of benzoyl protection of the phenylhydrazone resulted in the more efficient formation of the indole system. |
Benzyl |
|
| The use of benzyl as a protecting group led to a more efficient synthesis as opposed to benzoyl due to the fact that benzyl is easily added and removed. Benzyl is added by a simple Sn2 reaction and removed by mild catalytic hydrogenation. Benzoyl, however, is added with a strong base and deprotected using a nucleophile. Specifically looking at our molecule –(-) 52, deprotection of benzyl with catalytic hydrogenation would not affect other functional groups present in the compound as there are no non-aromatic double bonds to break. Deprotection of benzoyl with a nucleophile, however, presents the possibility of a nucleophilic attack on the electrophilic carbon of the carbonyl group. Such interference with other functional groups would hinder the efficiency of the reaction and lead to a lower yield of the desired product. |
Benzoyl |
Thor Home Experimental Mechanism HNMR Correlations References About Us SSG Home