
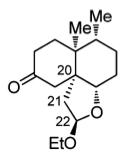
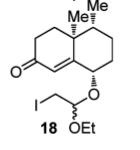
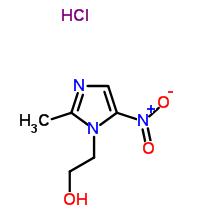
Tricyclic ketone (14): A solution of n-Bu3SnH (5.52 g, 5.10 mL, 19.0 mmol) in toluene (20 mL) was added dropwise over 2 hours at 80°C to a stirred solution of todoacetal 18 (3.74 g, 9.53 mmol) and AIBN (780 mg, 4.75 mmol) in degassed toluene (30 mL). The mixture was then cooled to 22°C before the solvent was evaporated under vacuum. Flash chromatography was done with the residue using EtOAc/petroleum ether (1:20 --> 1:5)as eluent, to give tricyclic ketone 14 (1.29 g, 51%) and its C11 epimer (0.56 g, 22%) both as colorless oils. The minor isomer so obtained was dissolved in CH2Cl2 (20 mL), and ethanolic HCl (0.50 mL, 2.0 M) added at 22°C. The mixture was then vigorously stirred for one hour before being quenched with saturated aqueous NaHCO3 solution (100 mL). After being extracted with EtOAc (3 x 100 mL), the combined organic phase was washed with brine (50 mL) and dried with MgSO4, filtered via gravity filtration, and concentrated under vacuum. The residue obtained was further purified by flash column chromatography with EtOAc/petroleum ether (1:20 --> 1:5) to give a second portion of tricyclic ketone 14 (0.35 g, 14%), making the combined yield of 14 from 18 65%.
Glossary
Click the glass slipper to go back to the homepage!