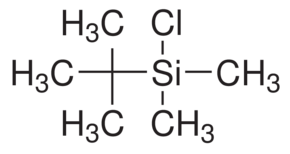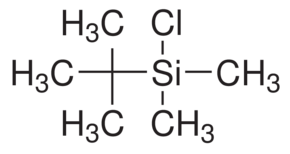
Better known as TBSCL, a organosilicon compound, this particular compound falls under a class of compounds known as silyl chlorides, which play a large role in many organic syntheses. The use of TBSCL here and in most other syntheses is as a protecting group, which allows reactions on other parts of the molecule to take place. TBSCL is bonded to imidazole, which kicks off the chlorine, then the hydroxyl group bonds with the silicon, dropping off the imidazole. This forms the silyl ether, which are used as protecting groups for alcohols, hence the name of the first step being Silylation.