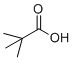
It is used as a catalytic acid in this reaction. Its conjugate base helps palladium couple 3,4-(methylenedioxy) iodobenzene with S1. Pivalic acid is colorless and odoriferous solid at room temperature. However, its melting point is 35 °C, which is fair close to room temperature. Esters of pivalic acid have high resistivity to hydrolysis and high thermal stability. Because of these unique features of pivalate esters, they are often used to form polymers.
Hazards:
Like most carboxylic acids, pivalic acid is weakly toxic.
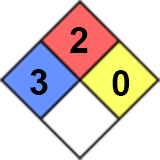
Health (blue) 3:Short exposure could cause serious temporary or moderate residual injury.
Flammability (red) 2: Must be moderately heated or exposed to relatively high ambient temperature before ignition can occur.
Instability/Reactivity (yellow) 0: Normally stable, even under fire exposure conditions, and is not reactive with water (e.g. helium).

