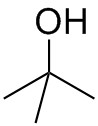

Together with heat, tert-butyl alcohol is used to generate reactive palladium (0) in this reaction. Generally, tert-butyl alcohol is used as a common solvent. As a tertiary alcohol, tert-butyl alcohol is more stable to oxidation. Its conjugate base, tert-butoxide is a strong base. Since it is bulky, tert-butoxide normally does not participate in nucleophilic substitution, such as in a Williamson ether synthesis or an SN2 reaction.


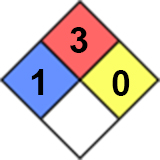
Health (blue) 1: Exposure would cause irritation with only minor residual injury (e.g. acetone).
Flammability (red) 3: Liquids and solids (including finely divided suspended solids) that can be ignited under almost all ambient temperature conditions.
Instability/Reactivity (yellow) 0: Normally stable, even under fire exposure conditions, and is not reactive with water (e.g. helium).