Main


Gutekunst, W.R.; Gianatassio, R.; Baran, P. S. Agnew. Chem. Int. Ed. 2012, 51, 7507-7510.
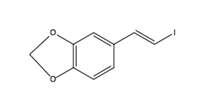
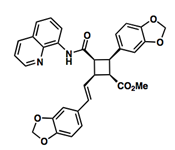
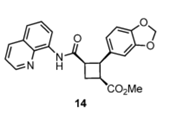
Pipercyclobutanamide A can be synthesized through a 6-7 step procedure and then a structural rearrangement that includes Csp3-H Arylation and Olefination reactions. The following reaction involves the C-H olefination reaction of molecule 14 with 3,4-(methylenedioxy)styrenyl iodide to give molecule 16. This reaction gives an all cis configuration that proceeds to be converted into a trans configuration for the product.
Back to Nick's homepage
Mechanism
Complete Arrow Pushing Mechanism:



HNMR
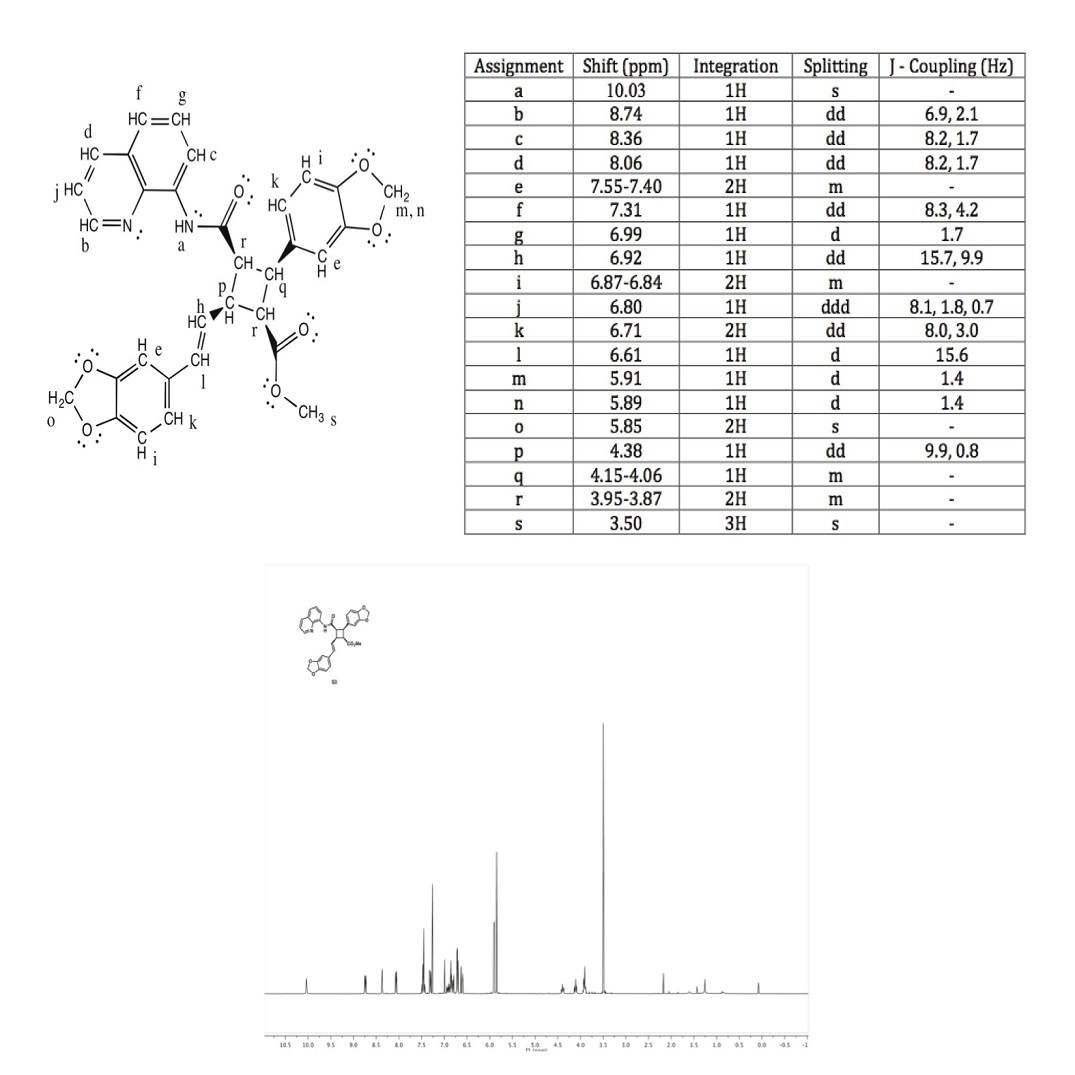
a: The broad singlet with just one hydrogen at 10.03 ppm indicates that it is bonded to a halogen group, in this case the N-H group.
b: An aromatic hydrogen shift typically falls around 6.5 - 8.5 ppm. The electronegative Nitrogen in the ring deshields these hydrogens, making its shift the furthest downfield.
c, d, f, g, j: Aromatic hydrogen shifts typically fall around 6.5 - 8.5 ppm. They also have corresponding coupling constants, indicating that these protons are coupled in the same ring.
e, i, k: The shift at 7.55 - 7.40 ppm is an aromatic hydrogen. There are two hydrogens because they are almost identical on the two different rings.
h: The shift at 6.92 ppm has a coupling constant of 15.7 Hz for one of its J values. Therefore, it is likely one of the trans coupled protons. It is also has another 3-bond neighbor, indicated that it is the trans coupled proton that is also neighbored with p, J = 9.9 Hz
l: The shift at 6.92 ppm has a coupling constant of 15.7 Hz for one of its J values. Therefore, it is likely one of the trans coupled protons. This proton only has one 3-bond neighbor so it is the trans coupled proton that is only a doublet, not a doublet of doublets.
m, n: The shifts 5.91 and 5.89 are so close together, indicating that they are diastereotopic hydrogens, that are two-bond neighbors, bonded to the same carbon.
o: The shift at 5.85 is a singlet, as there are no neighbors. There are 2 hydrogens there, giving it a 2H integration, and the oxygen is deshielding these hydrogens, shifting the peak downfield.
p: The shift at 4.38 is coupled with proton h, because they have the same J values. It is also a doublet of doublets because of the two differing neighbors and coupling constants.
q: The shift at 4.15 - 4.06 ppm has a very close shift to p because they are bonded to almost identical groups. There is only one hydrogen and it is a multiplet because of the various similar interacting neighboring hydrogens.
r: The shift at 3.95 - 3.87 ppm corresponds to two different hydrogens. The two hydrogens marked r are almost identical in their bonding with carbonyl groups and their coupling is a multiplet due to the various similar interacting neighboring hydrogens.
s: The shift at 3.50 ppm corresponds to the only methyl group in the compound. It is shifted downfield due to the CO2 group attached to the same carbon and appears as a singlet.
Experimental
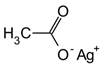
Molecule 14 (480 mg, 1.19 mmol), Pd(OAc)2 (40 mg, 0.18 mmol, 0.15 eq), AgOAc (298 mg, 1.78 mmol), and 3,4-(methylenedioxy)styrenyl iodide (2.62 g, 10.56 mmol, 3 eq) were mixed in a tube. Under ambient conditions, 5.9 ml of toluene (0.2 M) was added, the tube was sealed, and placed into an oil bath for 10 hours at 80 oC. The solution was cooled to room temperature and DCM (10 ml) was added. The solution was filtered through celite and concentrated under vacuum. Silica gel chromatography (3% to 4 % Ethyl acetate in toluene) purified the orange oil, giving molecule 16 (386 mg, 59%) as yellow foam.

References
Mechanism Related Sources:
These were cited by our article:
Barton D. H. R.; Hesse, R. H.; Wilshire, C.; Pechet, M. M. J. Chem. Soc. Perkin Trans. 1, 1977, 9, 1075 - 1079.
Pelletier, G.; Bechara, W.S; Charette, A. B. J. Am. Chem. Soc., 2010, 132, 12817 - 12819.
Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc., 2005, 127, 13154 - 13155.
“Highly Regioselective Arylation of sp3 C-H Bonds Catalyzed by Palladium Acetate,” was an incredibly helpful journal article to explain our mechanism. Pyridine tethered to an aromatic compound as a directing group, which creates a complexation between Palladium and Nitrogen, is observed in an analogous fashion in, “Sequential Csp3H Arylation and Olefination: Total Synthesis of the Proposed Structure of Pipercyclobutanamide A*.” In addition, the reaction conditions in both articles are similar, allowing us to explain our mechanism in the most detail possible.


This article does an arylation of methylene C-H bonds using palladium (II/IV) catalysts. Our paper was useful because of a similar use of a palladium catalyst in a C-H bond olefination reaction.
Pearson, R.; Zhang, S.; He, G.; Edwards, N.; Chen, G. Beilstein J. Org. Chem., 2013, 9, 891 - 899.
This article uses a picolinamide (PA) group as a directing group for a Pd-catalyzed C-H functionalization reaction. Our particular reaction also uses a picolinamide directing group for the reaction.
Wasa, M.; Chan, K.S.L.; Zhang, X.; He, J.; Miura, M.; Yu, J. J. Am. Chem. Soc., 2012, 134, 18570 - 18572.
This article referenced the insertion of the Pd into a C-H bond with the requirement of a directing group such as pyridine. Our reaction used a palladium catalyst with a similar directing group, picolinamide, to undergo a C-H ligand directed reaction.
Leading Question
Q: Explain why 16 is formed as a single diastereomer.

A: The reaction with the Pd catalyst at the C-H bond is more stable in the transition state that contains the very bulky substituents in an anti-position, because of sterics. This suggests that in order for the C-H bond to be cleaved, there must be a minimum dihedral angle between the C-H bond and the Pd-OAc bond that exists in the transition state. This transition state subsequently allows the reaction to continue, thus forming the single diastereomer in our product 16.
Giri, R.; Lan, Y.; Liu, P.; Houk, K.N.; Yu, J. J. Am. Chem. Soc., 2012, 134, 14118 - 14126.
About Us
Evan Dahan

Evan Dahan was orginally cast to play the role of Jesse Pinkman in America's hit T.V. series, "Breaking Bad." Unfortunately, Aaron Paul's late audition tape won the hearts of the casting agents. This caused Evan Dahan to have a complete mental breakdown and drove him to fully emulate Jesse Pinkman. Last year, Evan's meth cooking operation was busted, and he is now serving a life sentence without parole at the Penitentiary of New Mexico.
Audrey Niemchick

Audrey is a 6 time award winning play-doh sculptor by day and crime fighting Batgirl by night. She prowls the streets of Gotham protecting the citizens from the wrath of evil-doers while bringing delight with her doughy creations. She also enjoys smiling, puppies and long walks on the beach.
James Rhee

Born and raised in south Detroit, James has worked for NASA for 10 years, has traveled to Mars, and established surviving colonies. Currently, he studies organic chemistry at the University of Michigan, Ann Arbor to discover the chemical compositions of possible extraterrestrial life on potential habitable planets. His ambitions are to establish a base on Jupiter's moons and visit pluto in the near future. In his free time, he loves to play Super Smash Brothers Melee/Brawl and basketball with his family and friends.
 acetate.png)