EXPERIMENTAL
Mouse over the underlined stuff!
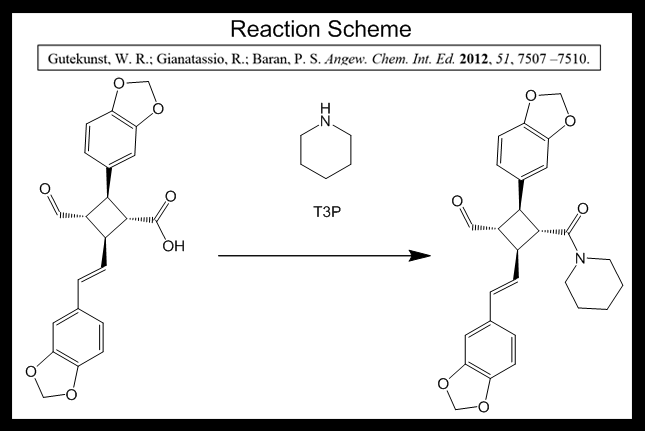
Crude 19 (0.686 mmol) was dissolved in dichloromethane (DCM, 7.0 mL) and piperidine (0.20 mL, 2.06 mmol, 3 eq) was added at room temperature. This allows for the first step of the reaction to occur, in which piperidine deprotonates the carboxylic acid on 19. T3P (50% w/w solution in ethyl acetate, 0.612 mL, 1.03 mmol, 1.5 eq) was added dropwise, and the reaction mixture was stirred for 15 minutes while the reaction completed. 3M HCl (3 mL) was added to quench the reaction, using up the remaining piperidine in order to remove it from solution. The layers were separated in a liquid-liquid extraction, where the aqueous layer contained the acid-base reaction impurities and the reacted T3P and the organic layer contained molecule 20 and unreacted molecule 19. The aqueous layer was washed with DCM (3 x 5 mL), the combined organics were washed with brine to neutralize any remaining HCl, and then the organic layers were dried over sodium sulfate and the sodium sulfate was removed by gravity filtration. After concentration in vacuo (evaporating off excess DCM), the orange foam was purified by column chromatography (4:1 hexanes:ethyl acetate) to give 20 as a white foam (129.3 mg, 40% over 3 steps). Column chromatography would purify 20, as any unreacted 19 would elute more slowly than 20 due to the presence of an –OH group in 19 - this increases the polarity of 19 relative to 20 and increases its hydrogen bonding capability, making it pass through silica gel more slowly. When this three-step sequence was performed on 114 mg of S3, a 45% yield was obtained.






