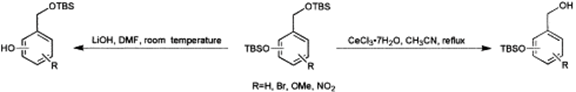
Agent Jones found some vital information in Hatcher Graduate Library... ReferenceDiscussion of a journal article relating to the Conversion of Molecule 15 to 16: Ankala, S. V.; Fenteany, G. Tetrahedron Lett. 2002, 43, 4729-4732. Ankala and Fenteany’s journal article presents a method for the selective deprotection of alkyl or aryl sillyl ethers. The technique was discovered in 2002. This discovery is essential for the conversion of compound 15 to compound 16. The use of cerium trichloride has the advantages of being inexpensive, non-toxic and as a mild Lewis-acid, a safer, milder reagent, than alternatives such as hydrogen fluoride. Compound 15 has two trimethylsilyl groups that require deprotection, however, the tert-butyl carbamate must be unaffected. Through the use of cerium trichloride, selective deprotection of the trimethylsilyl group can occur while leaving the tert-butyl carbamate which protects the nitrogen unaffected.
Citation #1: Gopinath, P.; Nilaya, S.; Muraleedharan, K. M. Org. Lett. 2011, 13, Citation #2: Wang, B.; Sun, H.; Sun, Z. J. Org. Chem. 2009, 74, 1781-1784. Citation #3: Yan, H.; Oh, J.-S.; Song, C. E. Org. Biomol. Chem. 2011, 9, 8119-8121. |
|---|