H-NMR for Compound 15
C-NMR
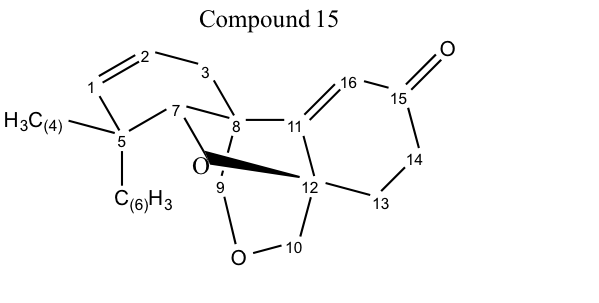
C1: This peak appears at approximately a 120 ppm shift, because it is a standard sp2 carbon. It is shifted farther than C2 because it’s neighbor is a quaternary carbon, while C2’s neighbor is only a secondary.
C2: This peak appears at approximately a 120 ppm shift, because it is a standard sp2 carbon. It is shifted less than C1 because it’s neighbor is only a secondary carbon, while C1’s neighbor is a quaternary carbon.
C3: This peak appears far upfield because it is a sp3 secondary carbon. The only peaks that appear farther upfield are primary carbons 5 and 6, and then secondary carbon 13. Carbon 3 appears farther downfield than carbon 13 because it is closer to a pi system.
C4: This peak appears far upfield because it is sp3 hybridized and is a primary carbon. It is not as far upfield as the axial carbon (carbon 6) because it is in the equatorial position which means it is in the deshielding region of the C-C anisotropy.
C5: This peak appears fairly far upfield because it is sp3 hybridized. It is farther downfield than some of the other sp3 hybridized carbons because it is a quaternary carbon.
C6: This peak appears the furthest upfield because it is a sp3, primary carbon. It is more upfield than C4 because it does not reside in the deshielding region of the C-C anisotropy.
C7: This peak appears in slightly over 70 ppm, because it is bonded to an electronegative oxygen atom that causes deshielding. It’s attachment to two other carbons further increases the amount of deshielding from the normal oxygen binding of a primary carbon.
C8: This peak appears fairly upfield because it is a sp3 carbon that is not bound to any electronegative atoms. It is a quaternary carbon and therefore appears farther downfield than the secondary and primary carbons.
C9: This peak appears at approximately 70 ppm because it is a secondary carbon bound to an electronegative atom (oxygen). It appears farther upfield than C10 because it is farther away to another electronegative atom on the other side.
C10: This peak appears at approximately 75 ppm because it is a secondary carbon bound to an electronegative atom (oxygen). It appears farther downfield than C9 because it is closer to another electronegative atom on the other side.
C11: This peak appears the second most upfield because it is a sp2 hybridized carbon that is bound to 3 carbons. This larger amount of substitution leads to deshielding and a larger ppm shift.
C12: The peak appears in the middle of the spectrum because it is sp3 hybridized, but then is bonded to an electronegative oxygen atom. It is also bonded to three other carbons which further increases the amount of electron withdrawing and therefore deshielding that occurs.
C13: This peak appears far upfield because it is a sp3 secondary carbon. The only peaks that appear farther upfield are primary carbons 5 and 6. Carbon 13 appears farther upfield than carbon 3 because carbon 3 is closer to a pi system.
C14: This peak appears fairly far upfield because it is a sp3 hybridized secondary carbon. Out of all of the sp3 secondary carbons that are not bonded to an oxygen, this carbon peak appears the most downfield because it is next to a carbonyl group.
C15: This carbon peak appears the most downfield at approximately 198 ppm because it is associated with a ketone carbonyl. The pi system with the electronegative/electron withdrawing oxygen creates a lot of deshielding and thus a greater ppm value.
C16: This peak is the third most upfield, because though it is not bonded to an electronegative atom it is a part of a pi system and is next to an electron withdrawing carbonyl group.