Beware: What you are looking at is top secret information from the Fire Nation. Alkene Metathesis is a technique that only the wisest from the fire nation are capable of mastering. Dr. Schindler, who has placed an immense amount of trust in Montgomery, is willing to teach him the art of Alkene Metathesis.

What is metathesis?
Metathesis is the formation of a product that has exchanged bonds between starting materials. It is a subset of double displacement reactions.
Examples of metathesis reactions:
What is alkene metathesis?
Alkene metathesis, also known as Olefin metathesis, was the subject of the 2005 Nobel Prize in Chemistry. The reaction involves the rearrangement of carbon-carbon double bonds, through the use of metal catalysts, such as the Grubbs Ru catalyst, Schrock W, Mo, and Re catalysts, and titanocene-based catalysts. Note: Olefin is merely another term for alkene.
Mechanism:
The most commonly accepted mechanism of the Olefin metathesis involves the formation of a metallacyclobutane intermediate, as seen in the below diagram:
The Olefin metathesis reaction has very low stereospecificity (although, in some circumstances, the E configuration is preferred).
Note: LuRn=CH2 is a simplified drawing of the Ruthenium Grubbs catalyst. The catalyst used in the reaction is recreated at the end of the reaction (RuLn=CH2). “M” in the mechanism refers to the metal present in the catalyst for Olefin Metathesis (usually Ru, W, or Mo).
Click here to view the alkene metathesis mechanism involved in the formation of Batzelladine.
Real World Applications:
“Using chemistry we started out just by having fun, [but the result] is, now it is being used to make tank armor.” – Dr. Robert Grubbs.
Olefin metathesis is used to form polymers to create materials for bathroom sinks, stronger baseball bats, tank armor, Hepatitis C and Osteoporosis drugs, candles and for pest control.
In the orgotar world, mastering alkene metathesis means you are one of the strongest firebenders on the planet, because alkene metathesis is so useful. The founder of Alkene Metathesis, Firebending Master Grubbs, won the Nobel Orgotar Prize in 2005.
Types of Alkene Metathesis:
1. Cross metathesis:
The transalkylidenation of two terminal alkenes under release of ethene, catalyzed by ruthenium carbenoids (Grubbs Catalyst). Two main issues associated with cross metathesis include:
a. Must control homodimerization (see below diagram) in order to obtain useful yields
b. Generally prefers E isomer (hard to obtain Z isomer). However, E/Z ratios are also hard to control and predict.
2. Ring closing metathesis (RCM):
Involves the intramolecular metathesis of a diene to form a cyclic alkene. A general mechanism for the RCM can be seen below:
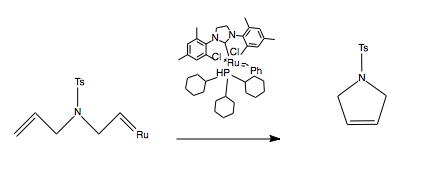
Ring closing metathesis requires the ring formed not to be strained. An example reaction of a RCM reaction can be seen below:
3. Ring-opening metathesis polymerization (ROMP):
Ring opening of strained cyclic olefin to give open chain metal carbene provides driving force for ROM. A general mechanism for the ROMP can be seen below:
Ring Opening Metathesis requires a strained ring to proceed. Follows cross metathesis. An example reaction of a ROMP reaction can be seen below:

Catalysts:
The Grubbs and Schrock catalysts were developed independently in the 1990s. The Schrock catalyst was commercialized in 1990 and is molybdenum(IV)- and tungsten(IV)-based while the Grubbs catalyst (first generation) was published in 1992 and makes use of ruthenium(II) carbenoid complexes. In 1999 a second generation Grubbs catalyst was developed that is stable when exposed to moisture and air with an effectiveness 100 to 1000 times that of the first generation.
