Benzyl ether hydrogenolysis: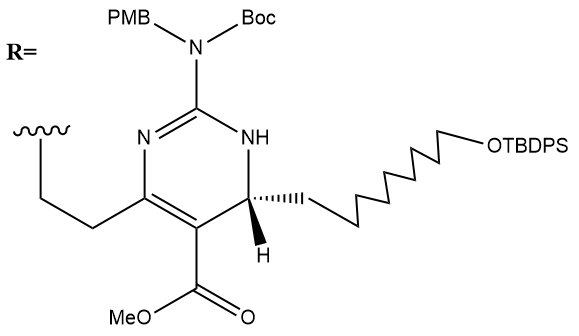
(a.k.a. Benzyl Removal/Deprotection)
P-Methoxybenzyl Removal: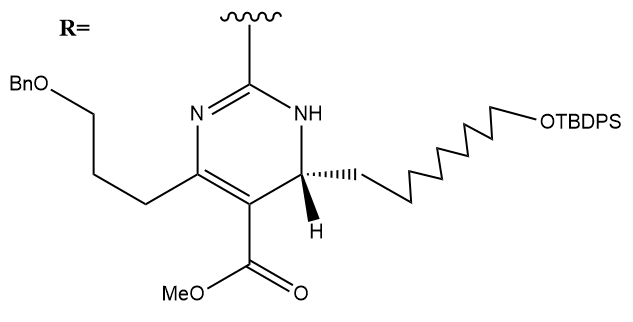
(P-Methoxybenzyl Deprotection)
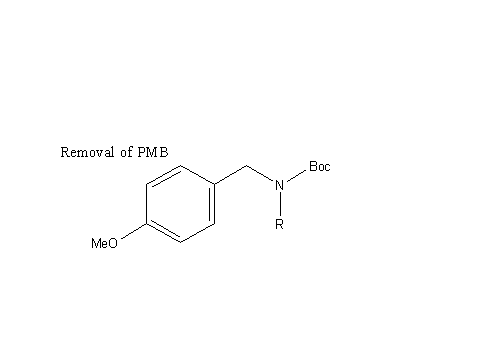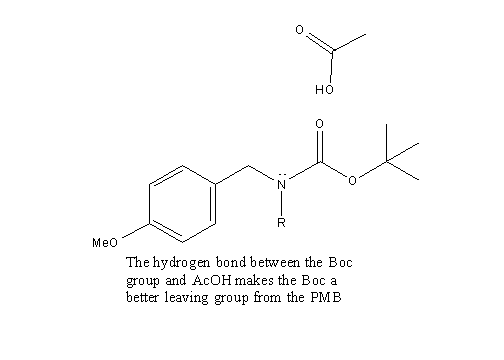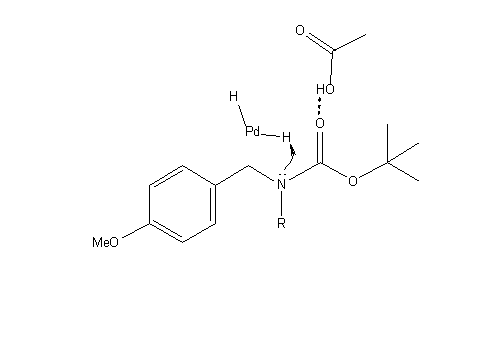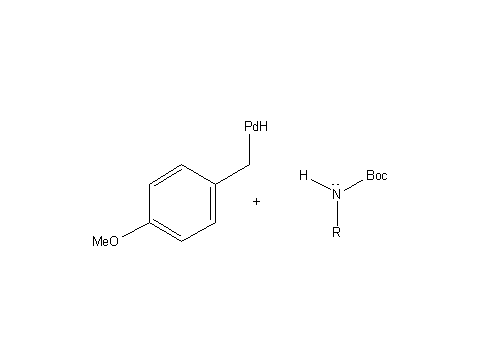
AcOH is used to facilitate the deprotection mechanism. The AcOH hydrogen bonds to the carbonyl of the BOC group, thereby making it easier for the p- methoxybenzyl to leave from the molecule.
The last chairbender, Dr. Coppola welcomes Montgomery! He is the last remaining chairbender and is willing to teach him hydrogenolysis and deprotection for the simple promise that Montgomery will carry on the ways of his people.
In addition to hydrogenation, the addition of H2 and Pd(OH)2 leads to benzyl ether hydrogenolysis and p methoxybenzyl removal from the guanidine (both of which are deprotecting mechanisms (from the air nomads), as described below:
The Basics of Deprotection:
Deprotection is merely the process of removing a protecting group. Two protecting groups are removed in the our mechanism: benzyl ether and p-methoxybenzyl. The AcOH is used to facilitate the deprotecting process.
As explained above, this step of the reaction proceeds through the deprotection of two different locations on the molecule. The first step in both processes involves the hydrogenation of Pearlman’s catalyst to form PdH2, as seen below:
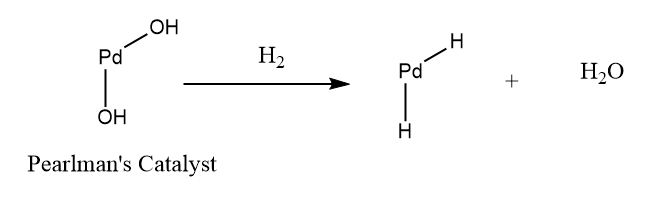
As mentioned by Dr. Wolfe of the Air Nomads, this mechanism is beyond the scope of Chem 215H/216H and therefore, will not be displayed the website.
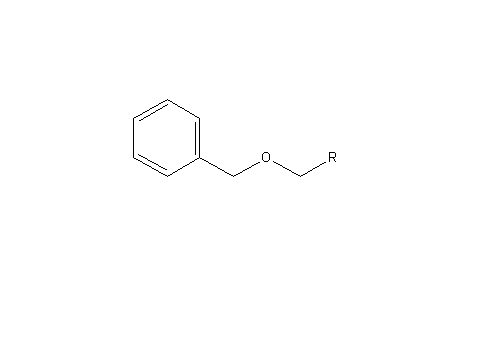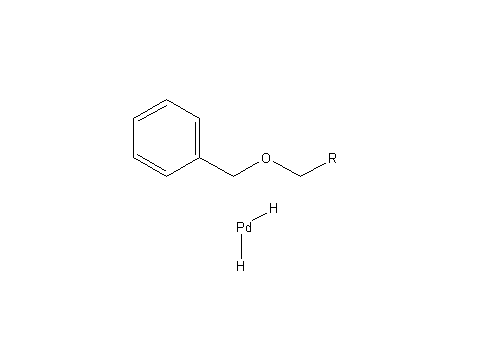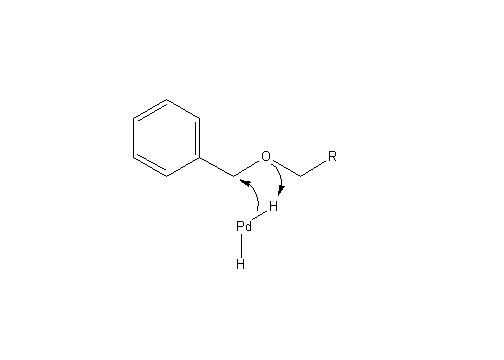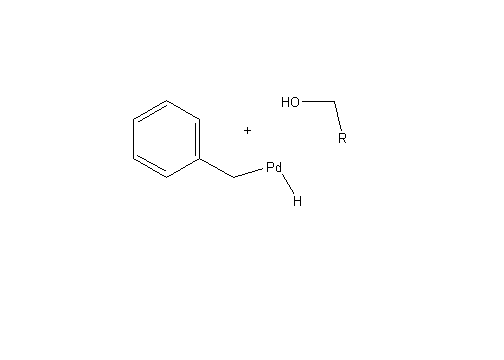
After this reaction has occurred it is possible for both protecting groups to be removed by hydrogenolysis. This process is similar to hydrogenation but has the ability to break C-C and C-heteroatom bonds. In the case of this reaction, hydrogenolysis breaks O-C and N-C bonds to remove the protecting groups.