References:
Throughout his journey, Montgomery referred to these sources for helpful and relevant information.
Main Paper:
Hughes, D. L. Org. Prep. Proced. Int. 1996, 28, 127-164.
This paper outlines the Mitsunobu reaction, which allows a primary or secondary alcohol to be turned into an ester, thioester, phenyl ester, and other carbonyl derivatives. The mechanism employs triphenylphosphine as an electrophile that activates the oxygen on the alcohol as a good leaving group. A carboxylate ion is free to react as a nucleophile, doing SN2, and kicking off the activated oxygen leaving group. Nitrogen anion nucleophiles are also capable of doing SN2 in this reaction. The stereochemistry of the alcohol group is inverted as a result of the SN2. The reagent diethyl azodicarboxylate (DEAD) is used in the reaction as an electron source that gets protonated throughout the course of the reaction in order to prevent side reactions from occurring that will damage yield of the desired product. Additionally, the order that the products are added in is important when trying to minimize undesired side reactions. The Mitsunobu reaction is useful when synthesizing aryl ethers, but can yield undesired products from side reactions if the reaction is not done with care.
This is the Mitsunobu Reaction:
The paper explains that the first step in the Mitsunobu reaction involves a reaction betwen diisopropyl azodicarboxylate (DIAD) and PPh3, as seen below:
The rest of the Mitsunobu reaction follows a complex pathway, as shown below:
Paper 1: Synthesis and properties of O-glycosyl calix[4]arenes (calix sugars).
Dondoni, A.; Marra, A.; Scherrmann, M.-C.; Casnati, A.; Sansone, F.; Ungaro, R. Chem. Eur. J. 1997, 3, 1774–1782.
The paper involved in the synthesis of O-Glycosyl Calix through the Mitsunobu reaction (the reaction can be seen in the image below). D-mannofuranose and D-glucopyranose were introduced at the lower rim of the parent calixarene by glycosylation of the phenolic hydroxyl groups by means of a Mitsunobu reaction.
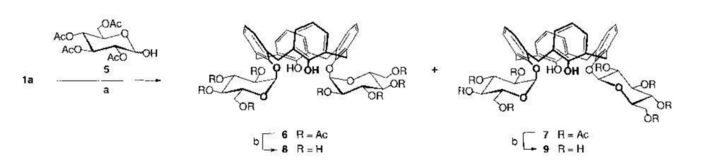
Paper 2: Design, Synthesis, and Characterization of Novel Tetrahydropyran-Based Bacterial Topoisomerase Inhibitors with Potent Anti-Gram-Positive Activity.
Surivet, J.; Zumbrunn, C.; Rueedi, G.; Hubschwerlen, C.; Bur, D.; Bruyère, T.; Locher, H.; Ritz, D.;Keck, W.;Seiler, P.; Kohl, C.;Gauvin, J.; Mirre, A.; Kaegi, V.; Dos Santos, M.; Gaertner, M,; Delers, J.; Enderlin-Paput, M.; Boehme, M. Journal of Medicinal Chemistry, 2013, 56, 7396-7415.
The paper proposed a synthesis for the creation of a tetrahydropyran-based antibacterial drug that are effective against infections caused by multidrug resistant pathogens. The drug is unique because it exhibits no target-mediated cross-resistance with fluoroquinolones. An intermediate (17) in this paper was constructed using the Mitsunobu reaction conditions to induce direct coupling of an alcohol (this reaction can also be seen in the below image).
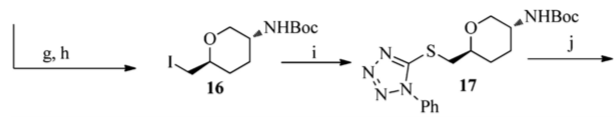
Paper 3: An effective approach to artificial nucleases using copper (II) complexes bearing nucleobases.
Wang, J.; Xia, Q.; Zheng, X.; Chen, H.; Chao, H.; Mao, Z.; Jia, L. Dalton Trans. 2010, 39, 2128-2136.
The paper proposes a complex that may be one of the most effective artificial nucleases that can catalyze double-stranded DNA hydrolytic cleavage. The authors of this paper were concerned that the Mitsunobu reaction would not be able to produce carbocyclic nucleosides from substituted cyclopentanols due to the low solubility of adenine (the original paper was referenced in order to confirm this fact). The specific reaction concerned can be seen in the image below.
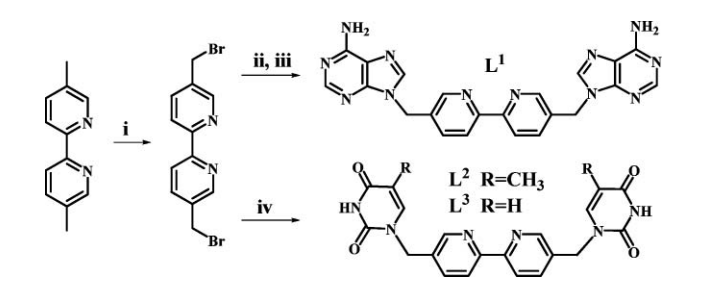
Other References Related to and Used toUnderstand this Mechanism:
Paper 4: Lippstreu, J. J.; Straub, B. F. J. Am. Chem. Soc. 2005, 127, 7444-7457
This paper cites Scholl in its analysis of the use of a first and second generation Grubbs Ruthenium catalyst in enyne metathesis.
Paper 5: Zhao, Y.; Truhlar, D. G. Org. Lett. 2007, 9, 1967-1970.
This paper cites Scholl in its explantation of why second generation Grubbs Ruthenium catalysts are 100-1000 times more effective than first generation Grubbs Ruthenium catalysts due to attractive noncovalent reactions.
Paper 6: Yao, Q.; Motta A. R. Tetrahedron Lett. 2004, 45, 2447-2551.
This paper cites Scholl in its production of a Hoveyda–Grubbs Ru catalyst which is derived from the second generation Grubbs catalyst. The purpose was to bind the derivative to polyethylene glycol to make it more recyclable and reusable in ring opening and closing reactions.