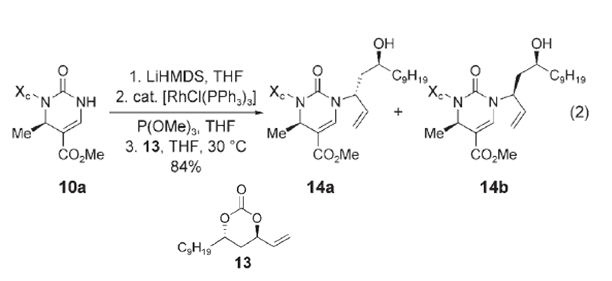
1. Lithium bis(trimethylsilyl)amide(LiHMDS)is a strong base that is not nucleophilic; this deprotonates the nitrogen on N-sulfonyl pyrimidine (10a).
2. The Wilkinson, [RhCl(PPh3)3], attacks the alkene on the cyclic carbonate pushing the double bond to the adjacent carbon, the bond between carbon and oxygen then breaks releasing a molecule of CO2 and protonating the remaining oxygen resulting in an OH group. At this time the the Wilkinson catalyst is bound to the alkane and also partially bound to the alkene giving the alkene a partial positive charge.
3. The newly free electron pairs on the nitrogen on Lithium bis(trimethylsilyl)amide (10a) attack the partial positive alkene pushing off the Wilkinson catalyst and pushing the alkene back and resulting in the final major product (14a).