
References: Takeda, T.; Tsuchida, T.; Fujiwara, T. Titanium tetrachloride promoted reduction of alkenyl sulfides using triethylsilane as a reducing agent. Chem. Lett. 1984, 13, 1219-1220.
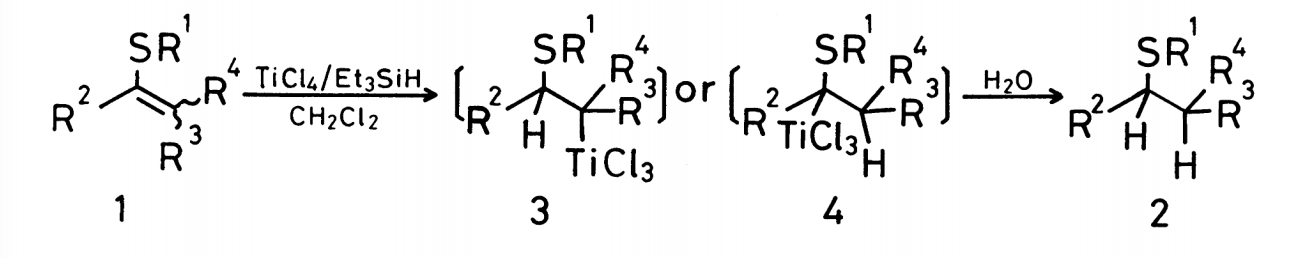
This experiment examined the combination of titanium tetrachlorine and triethyl silane in DCM as reducing agents. Specifically, they focused on alkenyl sulfides and found that those specific reaction conditions reduced the starting material to an alkyl sulfide. The reduction proceeded via an alkytitanium intermediate. This study was one of the first to examine titanium tetrachloride promoted reduction of alkenes with organosilanes (more specifically, Et3SiH). While the reduction examined here did not result in the deoxygenation of the starting material (as it does in our reaction), it is significant because it explains the reduction mechanism of these specific reaction conditions.
sheidy: Orfanopoulos, M.; Smonou, I. Selective Reduction of Diaryl or Aryl Alkyl Alcohols in the Presence of Primary Hydroxyl or Ester Groups by Etherated Boron Trifluoride-Triethylsilane. System. Synth. Commun. 1988, 18, 833-839.
This paper did not cite the aforementioned citation. We included this citation because it involves reductive deoxygenation using triethylsilane in DCM.
jessead: Gevorgyan, V.; Rubin, M.; Benson, S.; Liu, J-X.; Yamamoto, Y. A Novel B(C6F5)3-Catalyzed Reduction of Alcohols and Cleavage of Aryl and Alkyl Ethers with Hydrosilanes. J. Org. Chem. 2000, 65, 6179-6186.
The authors of this paper referenced the work of Orfanopoulos, M.; Smonou, I. as a source that provided information about the traditional reduction of alcohols and ethers using triethylsilane as a Lewis Acid.
jpwolfe:Hermann, J. M.; König, B. Reductive Deoxygenation of Alcohols: Catalytic Methods Beyond Barton-McCombie Deoxygenation. Eur. J. Org. Chem. 2013, 31, 7017-7027.
This paper referenced the Gevorgyan et al. paper for its use of B(C6F5)3 as a catalyitic hydride source to activate C-O bonds in combination with triethylsilane for reductive deoxygenation.
