Activities on the Deck (Leading Questions)
We've got all sorts of activities scheduled for this week, Today we're gonna develop a tutorial for our boatmates on the Horner-Wadsworth-Emmons reaction
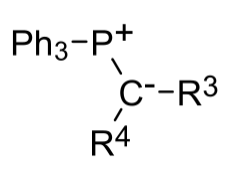
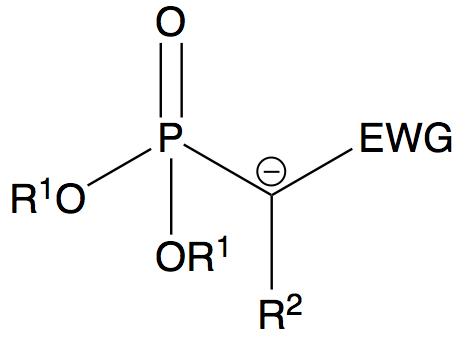
The Horner-Wadsworth-Emmons (HWE) reaction is a variation of the famous Wittig reaction, an essential in synthesis chemistry which produces an olefin and triphenylphosphine oxide from an aldehyde(or ketone) and a triphenyl phosphonium ylide. Instead of triphenyl phosphonium ylides, the Wittig reaction is modified to use phosphonate stabilized carbanions instead, which are more nucleophilic and less basic in nature. The reaction occurs between the stabilized phosphonate carbanion and an aldehyde (or ketone) to form an alkene. This reaction is generally (E)-selective, and unlike phosphonium ylides the dialkyl phosphate salt byproduct is easily removed by aqueous extraction making it more attractive.
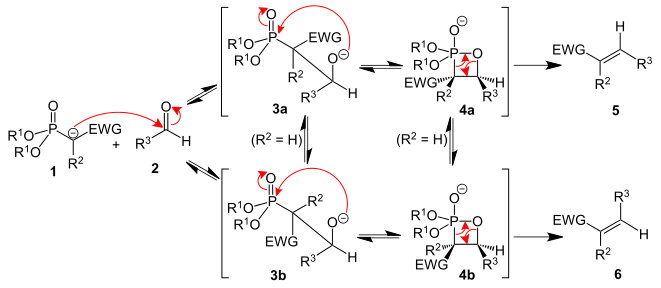
The reaction begins with nucleophilic attack of the aldehyde by the carbanion, followed by formation of an intermediate via intramolecular nucleophilic attack of the phosphorus. This intermediate eliminates the phosphate group, producing the alkene product. For the synthesis of molecule 8 the Roush-modified HWE is used, which simply uses milder bases for the reaction.
Arts and Crafts anyone?
