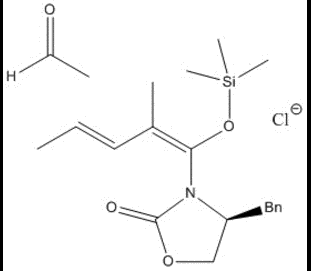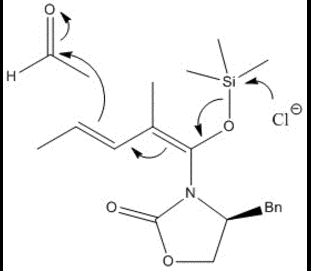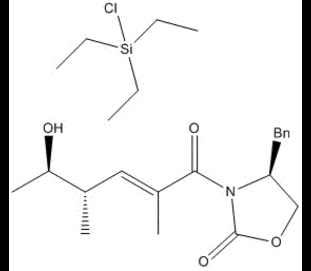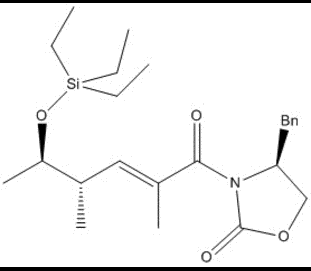

The first step in the reaction sees the anionic chlorine attack the trimethyl-silicon group, causing a carbon-oxygen double bond to form.
This, in turn, pushes the adjacent double bonds to the "left" of the molecule, where the last double bond then attacks the acetaldehyde electrophilic carbon.
The following tetrahedral intermediate is protonated, and after some rotation the molecule appears as it does in the final steps of the reaction.
The oxygn then acts as the nucleophile in an SN2 substitution of the chlorine on a triethyl silicon group, and after a deprotonation, is the product of this step in the synthesis of lactimidomycin.