1.) Use of 1 equivalent of 1-n-butyl-3-methylimidazolium iodide or other halides in vials, 1 equivalent of a strong acid at 0°C and allowed to stir.
.png)
(Reference: Ren, R. X.; Wu, J. X. Org. Lett. 2001, 3(23), 3727 – 3728. DOI:10.1021/ol016672r)
1.) Use of 1 equivalent of 1-n-butyl-3-methylimidazolium iodide or other halides in vials, 1 equivalent of a strong acid at 0°C and allowed to stir.
.png)
(Reference: Ren, R. X.; Wu, J. X. Org. Lett. 2001, 3(23), 3727 – 3728. DOI:10.1021/ol016672r)
2.) An alkyl chloride would have to be created first and then a substitution with Iodine.
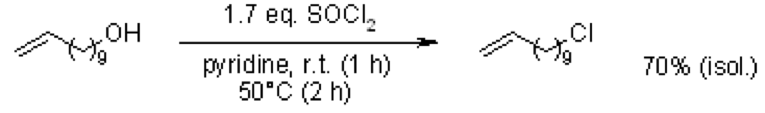

(Reference: Baughman, T. W.; Sworen, J. C.;Wagener, K. B. Tetrahedron, 2004, 60, 10943-10948. DOI: 10.1016/j.tet.2004.09.021)
3.)
Treatment of primary and secondary alcohols with MeSCH═NMe2+ I− gave the corresponding alkyl iodide in relatively decent yield.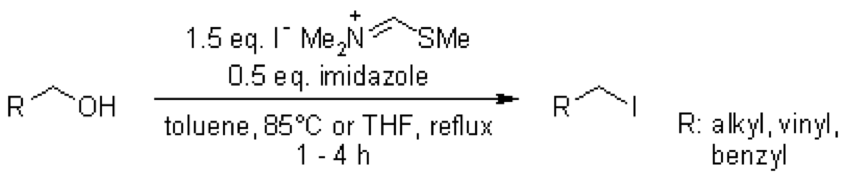
HTML QUESTION
Provide three other sets of reaction conditions that could have been used for the conversion of the alcohol to the alkyl iodide.
CONDITIONS USED FOR YOUR ORIGINAL SYNTHESIS: PPH3 & I2 Complex with SN2 chemistry
(References: Ellwood, A. R.; Porter M. J. J. Org. Chem., 2009, 74, 7982-7985. DOI: 10.1021/jo901415n)