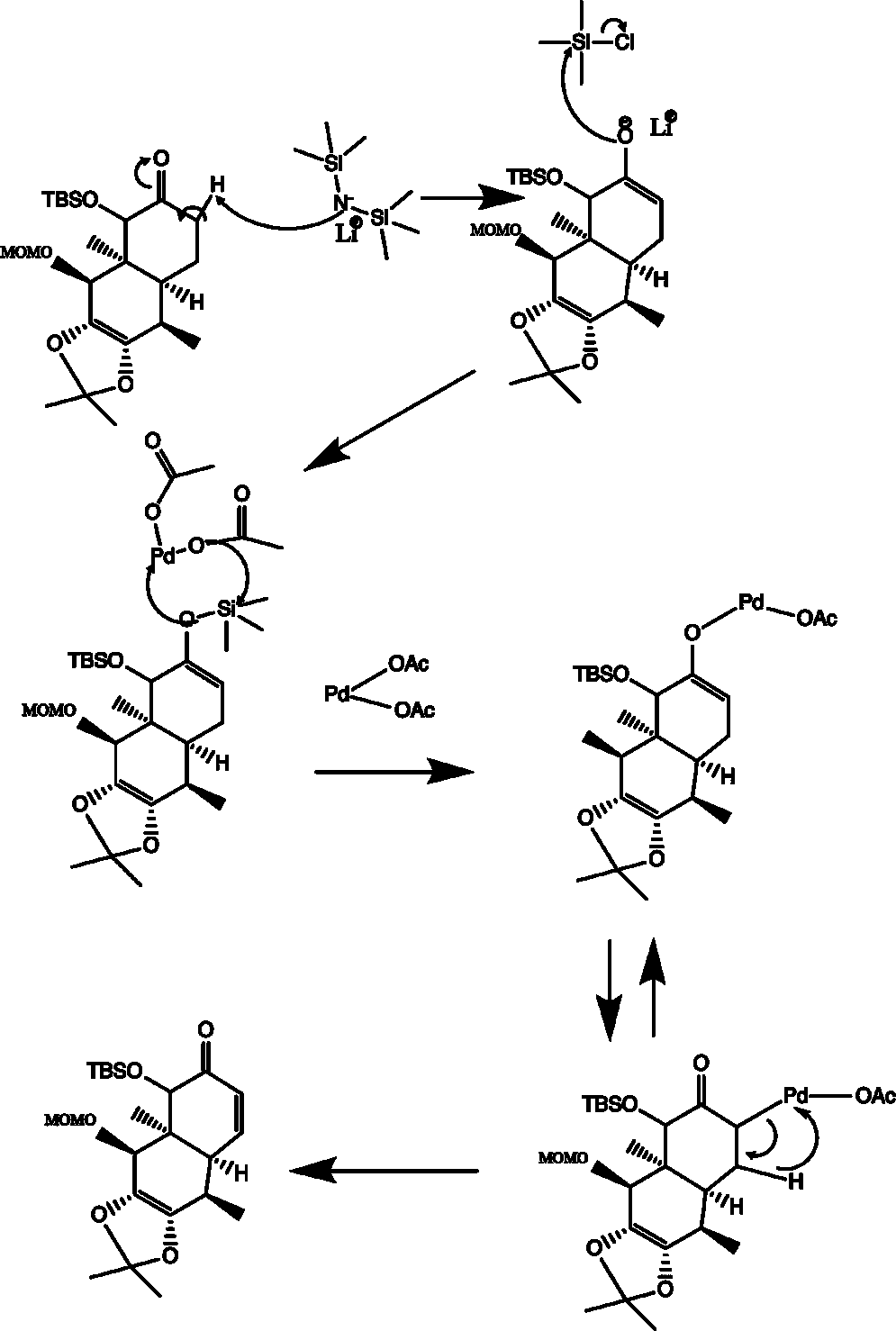
Mechanism of (+)-29→(−)-30

This step is an example of the Larock modification of the Saegusa-Itō oxidation. Lithium hexamethyldisilamide (LiHMDS) deprotonates the acidic proton adjacent to the carbonyl to form an enolate. Because it is a hindered base, it deprotonates at the less-substituted site (i.e. the site that does not have the OTMS group). The O-of the enolate attacks the silicon atom of trimethylsilyl chloride (TMSCl) and undergoes an SN2 reaction, forming a chloride ion and a silyl enol ether. The silyl enol ether is then treated with palladium (II) acetate, which coordinates with the silyl enol ether. The trimethylsilyl group decomplexes with the oxygen and removes an acetate group from the palladium. The species then undergoes a reaction similar to keto-enol tautomerization, in which the palladium complexes with the carbanion of the enolate. This is followed by a β-hydrogen elimination, forming the α,β-unsaturated ketone (30) along with acetic acid and elemental palladium.
Mechanism of (−)-30→(+)-31

This step is a typical copper-catalyzed conjugate addition of a Grignard reagent to an α,β-unsaturated ketone. First, two equivalents of the Grignard reagent complex with the cuprous ion to form a cuprous divinyl magnesium bromide complex, which is effectively a source of the nucleophilic vinyl anion. The viny anion attacks at the β-carbon of the ketone, forming an enolate. The enolate carbanion is protonated, forming a ketone (31).

