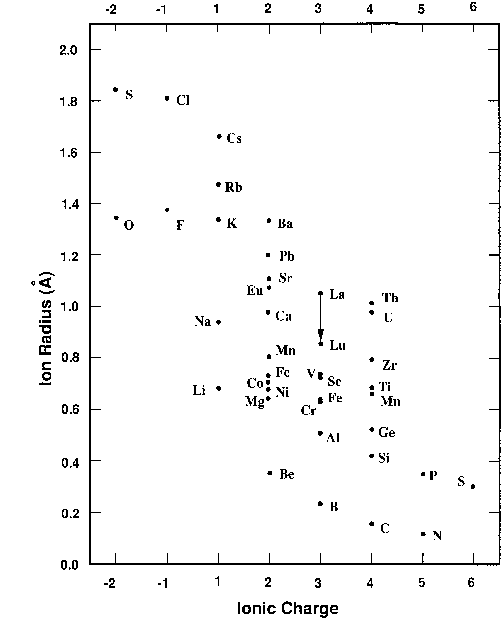
The radii of a variety of elements are plotted here as a function of their most common charge when they are undergo ionic bonding. The radii are given in Angstrom units (10-8 cm). Negative ions are plotted to the left. Note that sulfur is plotted both as a -2 and a +6.
This plot is useful for telling at a glance which ions can substitute for one another. Rare elements often are distributed among abundant minerals by subsituting for more common elements. For example, the radioactive isotope of rubidium, 87Rb typically substitutes for potassium, since the ions in their commin valence states have radii around 1.4 Angstrom units. Generally speaking, one ion will readily substitute for another if the radii are within about 15% of one another, and if the valences are not different by more than one unit. In the minerals albite (NaAlSi3O8) and anorthite (CaAl2Si2O8) there is a double substitution: sodium and one of the silicons in albite substitute for calcium and aluminum in anorthite. This is possible because the Ca and Na ions are similar in radii, and the Al and Si ions are also similar. Note that the valence differences just compensate. Na+ and Si4+ have the same positive valence as Ca2+ and Al3+.