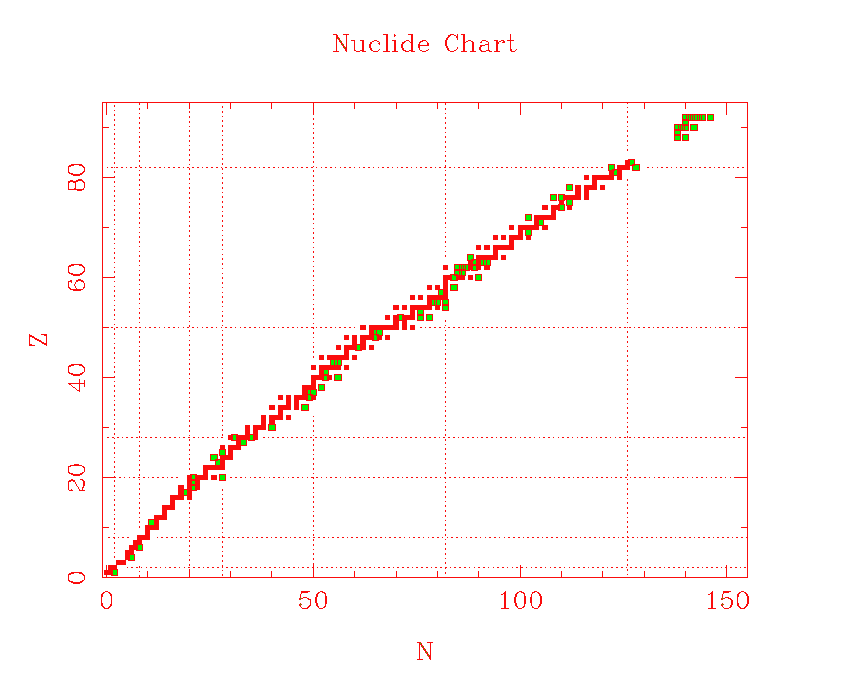

A Nuclide Chart is a plot of the number of protons (Z) vs. the number of neutrons (N), for stable and long-lived nuclides. Remember that the word "nuclide" means almost the same as "isotope." Stable nuclides are plotted as red squares. Some longer-lived radioactive isotopes are plotted as green squares with red outlines. The region of this plot where these nuclides fall is called the valley of beta stability. Physicists have investigated isotopes or nuclides that fall away from this valley, but the further away one goes, the shorter the lifetimes of the nuclides become. Generally, nuclides that are "off" the valley will emit either positive or negative electrons until they get to a value of Z and N represented by one of the black squares.
There is only positive charge in the nucleus. Nuclei would explode
if the strong nuclear force were not able to overwhelm the repulsive
electromagnetic force of the protons. This attractive nuclear force acts
only over very small distances (some 10-13 cm). For large nuclei,
the coulomb force begins to compete with the attractive nuclear force.
With smaller nuclei, equal numbers of protons and neutrons are very stable.
The light, so-called  -rich nuclei are multiples of
the 4He nucleus and are very stable. These nuclei are
12C, 16O, 20Ne, 24Mg,
28Si, and 32S.
-rich nuclei are multiples of
the 4He nucleus and are very stable. These nuclei are
12C, 16O, 20Ne, 24Mg,
28Si, and 32S.
As the nuclei become larger, more stable configurations contain a an excess of neutrons over protons. This causes the slope of the valley of beta stability to decrease. The effect increases toward the heavier nuclei. The heaviest stable nucleus is 209Bi, which has 126 neutrons, but only 83 protons. This is why, when heavy nuclei fission, the fragments are always neutron rich relative to their congeners with the same mass number A.
The dashed horizontal and vertical lines are for values of either Z or N of 2, 8, 10, 20, 28, 50, and 82. Nuclides with either Z or N equal to one of these numbers are called magic. Magic nuclei are relatively more stable than their neighbors. The phenomena is the nuclear analogue of shell closings in atoms--magic nuclei resemble noble gases in their stability. Physicists expected the nuclear shell closings to occur at the same numbers as atomic shells, and were at first baffled by the stability of nuclei with these special numbers of nucleons (protons or neutrons). They said the stability was magic. The phenomenon has been understood since the 1950's, and is related to the spin energy of nucleons, which is much greater than the corresponding energy of orbital electrons in atoms.
Return to index of figures and exercises.
Return to Cowley's HomePage.