A 400-L CSTR and a 100-L PFR are available to process 1.0 L of feed per second. The feed contains 41% A, 41% B, and 18% inerts. The irreversible gas-phase reaction
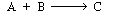
is to be carried out at 10 atm and 227°C. The rate of reaction
in g mol/L min is given below as a function of conversion:
min is given below as a function of conversion:

(a) What is the maximum conversion that can be achieved
with these two reactors connected in series? (Ans.: X C,P = 0.445, X P,C . = 0.515.)
(b) What would be the overall conversion if two 400-L CSTRs were connected
in series for the same feed and operating conditions? (Ans.: X = 0.595.)
(c) What would be the overall conversion if two 400-L CSTRs were connected
in parallel with half of the feed going to each reactor? (Ans.: X =
0.52.)
(d) What is the volume of a single tubular reactor necessary to achieve 60%
conversion if the molar feed rate is 2 g mol A/min? (Ans.: V
= 180 L)
(e) If the total pressure were reduced by a factor of 10, would the conversion
increase, decrease, or remain the same?
(f) Plot the rate of reaction and conversion as a function of PFR volume.
(g) Give a critique of the answers to this problem.